
����Ŀ����Ԫ�����Ŷ���̬�������Ļ�����ڹ�ũҵ����������Ҫ��Ӧ�á�
(1)ͬ����Ԫ���У��ȵ�Ԫ�صĵ�һ�����ܴ��Ԫ�ع���______________�֡�
(2)NF3�빯���ȿ��Ƶ�N2F2��
��NF3�ļ��ι���Ϊ______________��N2F2 �ЦҼ��ͦм��ĸ�����Ϊ_____________��
����֪��λ�ڵ������ڣ���пͬ�塣��̬��ԭ�ӵļ۵����Ų�ʽΪ_____________��
(3)H3N-BH3������Bԭ�ӵ��ӻ��������Ϊ_________���÷��ӵ��۷е������ߣ�ԭ����________________________��
(4)��B��N��FԪ����ɵ����ӻ������У�B��N��Fԭ�ӵĸ�����Ϊ1:1: 8,�����������ǵȵ�����,�û������е���������__________(�����ӷ���)��
(5)��������ṹ��ͼ��ʾ��
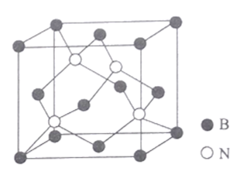
�ٸþ����к��е�����������Ϊ______________________��
��ͼ��Nԭ�ӵ��������Ϊ (![]() ��
��![]() ��
��![]() )��(
)��(![]() ��
��![]() ��
��![]() )��(
)��(![]() ��
��![]() ��
��![]() )��________��
)��________��
����֪BN���������������������ԭ�ӵĺ˼��Ϊacm��NAΪ�����ӵ�������ֵ.���ܶ�Ϊ_____________gcm-3 (�г�����ʽ����)��
���𰸡�2 ������ 3:1 5d106s2 sp3 H3N-BH3���Ӽ������� BF4- ���ۼ�(���Թ��ۼ�) (![]() ��
��![]() ��
��![]() )
) 
��������
(1)ͬһ����Ԫ�أ�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ�������Ԫ�أ�
![]() ������
������![]() ���ӽṹ���ƣ�
���ӽṹ���ƣ�![]() �ĽṹʽΪ
�ĽṹʽΪ![]() ��
��
![]() ��λ�ڵ������ڣ���пͬ�壬��֪пΪ��������Ԫ�أ��۵����Ų�ʽΪ
��λ�ڵ������ڣ���пͬ�壬��֪пΪ��������Ԫ�أ��۵����Ų�ʽΪ![]() ��
��
![]() ������
������![]()
![]() ������
������![]() ������λ����Bԭ�ӵ�2s�����3��p����ӻ����γ�4�����ۼ���
������λ����Bԭ�ӵ�2s�����3��p����ӻ����γ�4�����ۼ���![]() �����д���N��HԪ�أ����Ӽ���������
�����д���N��HԪ�أ����Ӽ���������
![]() �γɵĻ�������Fһ����-1�ۣ�B���ֻ����+3�ۣ�����NԪ��һ����+5�ۣ������γɵ������ӻ��������������Ϊ�ȵ����壻
�γɵĻ�������Fһ����-1�ۣ�B���ֻ����+3�ۣ�����NԪ��һ����+5�ۣ������γɵ������ӻ��������������Ϊ�ȵ����壻
![]() ��������ԭ�Ӿ��壬������ֻ���ڹ��ۼ���
��������ԭ�Ӿ��壬������ֻ���ڹ��ۼ���
![]() ��Ͼ�����4��Nԭ��������λ�÷�����
��Ͼ�����4��Nԭ��������λ�÷�����
![]() ��ȷ����������ɺ��������ٽ���ܶȹ�ʽ
��ȷ����������ɺ��������ٽ���ܶȹ�ʽ![]() ���㡣
���㡣
![]() ͬ�����������Ԫ�صĵ�һ�����ܳ��������ƣ�����Ԫ�ص�2p�ܼ���3�����ӣ����ڰ����ȶ�״̬����Ԫ�صĵ�һ�����ܸ���ͬ��������Ԫ�أ��ȵ�Ԫ�صĵ�һ�����ܴ��Ԫ��ΪF��Ne������2�֣�
ͬ�����������Ԫ�صĵ�һ�����ܳ��������ƣ�����Ԫ�ص�2p�ܼ���3�����ӣ����ڰ����ȶ�״̬����Ԫ�صĵ�һ�����ܸ���ͬ��������Ԫ�أ��ȵ�Ԫ�صĵ�һ�����ܴ��Ԫ��ΪF��Ne������2�֣�
(2)��![]() ������
������![]() ���ӽṹ���ƣ���ԭ�Ӻ���3�����ۼ���һ���µ��Ӷԣ����Կռ乹���������Σ�
���ӽṹ���ƣ���ԭ�Ӻ���3�����ۼ���һ���µ��Ӷԣ����Կռ乹���������Σ�![]() �ĽṹʽΪ
�ĽṹʽΪ![]() �����ݵ�������
�����ݵ�������![]() ����˫����һ����
����˫����һ����![]() ������һ����
������һ����![]() �����÷����к�3��
�����÷����к�3��![]() ����1��
����1��![]() ������ֵΪ
������ֵΪ![]() ��
��
![]() ��λ�ڵ������ڣ���пͬ�壬��֪пΪ��������Ԫ�أ��۵����Ų�ʽΪ
��λ�ڵ������ڣ���пͬ�壬��֪пΪ��������Ԫ�أ��۵����Ų�ʽΪ![]() �����̬��ԭ�ӵļ۵����Ų�ʽΪ
�����̬��ԭ�ӵļ۵����Ų�ʽΪ![]() ��
��
![]() ������
������![]()
![]() ������
������![]() ������λ����Bԭ�ӵ�2s�����3��p����ӻ����γ�4�����ۼ������Բ�ȡ
������λ����Bԭ�ӵ�2s�����3��p����ӻ����γ�4�����ۼ������Բ�ȡ![]() �ӻ���
�ӻ���![]() �����д���N��HԪ�أ����Ӽ����������������������������������ȷ��Ӽ������������Ը÷��ӵ��۷е������ߣ�
�����д���N��HԪ�أ����Ӽ����������������������������������ȷ��Ӽ������������Ը÷��ӵ��۷е������ߣ�
![]() �γɵĻ�������Fһ����-1�ۣ�B���ֻ����+3�ۣ�����NԪ��һ����+5�ۣ������γɵ������ӻ��������������Ϊ�ȵ����壬���Ƴ���������
�γɵĻ�������Fһ����-1�ۣ�B���ֻ����+3�ۣ�����NԪ��һ����+5�ۣ������γɵ������ӻ��������������Ϊ�ȵ����壬���Ƴ���������![]() ����������
����������![]() ��
��
![]() ��������ԭ�Ӿ��壬Bԭ�Ӻ�Nԭ���Լ��Թ��ۼ���ϣ�
��������ԭ�Ӿ��壬Bԭ�Ӻ�Nԭ���Լ��Թ��ۼ���ϣ�
![]() ͼ��Nԭ�ӵ��������Ϊ
ͼ��Nԭ�ӵ��������Ϊ![]() ��
��![]() ��
��![]() ����֪�����߳�Ϊ1��4��Nԭ�ӷֱ��������Խ����ϣ����Ͼ�����4��Nԭ��������λ�ÿ�֪��һ��ԭ�ӵ��������Ϊ��
����֪�����߳�Ϊ1��4��Nԭ�ӷֱ��������Խ����ϣ����Ͼ�����4��Nԭ��������λ�ÿ�֪��һ��ԭ�ӵ��������Ϊ��![]() ��
��
![]() ������Bԭ�Ӹ���Ϊ8��
������Bԭ�Ӹ���Ϊ8��![]() +6��
+6��![]() =4������Nԭ�Ӹ���Ϊ4�����к�4��BN���辧���߳�Ϊx������Խ��߳���Ϊ
=4������Nԭ�Ӹ���Ϊ4�����к�4��BN���辧���߳�Ϊx������Խ��߳���Ϊ![]() ����
����![]() ������1mol�����к�4molBN���ɼ��㾧�����ܶ�Ϊ
������1mol�����к�4molBN���ɼ��㾧�����ܶ�Ϊ![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ���й����ϼ�����ĵ��ĸ���ѧ����վ����̩ɽվ��Ϊ���ӳ���ѧ����վ������ʩʹ�������������豸������ǶһЩ������(M)������˵����ȷ����

A. ������M������ͭ������������Ӧ
B. ���ֱ�����������������������������
C. �ƿ�����վ����豸�ڳ�ʪ��������Ҫ������ѧ��ʴ
D. ��������ӵ������������������豸���Դ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ�����������ͽ��۾���ȷ���Ҵ��ڶ�Ӧ��ϵ����
ѡ�� | ʵ����� | ʵ������ | ���� |
A | ��NaOH��Һ��εμӵ�AlC13��Һ�������� | �Ȳ�����ɫ��״������������ܽ� | Al(OH)3�������������� |
B | NaHCO3��Һ��NaAlO��Һ��� | ���ɰ�ɫ���� | ���H+��������CO32->AlO2- |
C | ��ʢ��Na2SiO3����Һ���Թ��еμ�1�η�̪��Ȼ����μ���ϡ���������� | �Թ��к�ɫ����ȥ�����ְ�ɫ���� | �ǽ����ԣ�Cl>Si |
D |
| ��ɫ�����ȱ�Ϊ����ɫ�����Ϊ��ɫ | �ܶȻ�������Ksp(AgCl)>Ksp(AgBr)>Ksp(AgI) |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�pH ��Ϊ2������һԪ��HA��HB��1mL,�ֱ��ˮϡ��,pH ����Һ����ı仯������ͼ��ʾ������˵����ȷ����

A. HA�����Ա�HB��������
B. a����Һ�ĵ����Ա�c����Һ�ĵ�������
C. ������Һ��ˮϡ�ͣ����������ӵ�Ũ�ȶ���С
D. ��a��b������Һͬʱ�����¶ȣ���c(A-)/c(B-)��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��0.1 mol��þ�������������100 mL 2mol��L��1H2SO4��Һ�У�Ȼ���ٵμ�1 mol��L��1NaOH��Һ����ش�
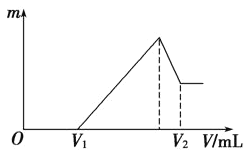
��1�����ڵμ�NaOH��Һ�Ĺ����У���������m�����NaOH��Һ�����V�仯��ͼ��ʾ����V1��120mLʱ���������ĩ��n(Mg)��________��V2��________mL��
��2�����ڵμ�NaOH��Һ�����У���ʹMg2����Al3���պó�����ȫ�������NaOH��Һ�����V(NaOH)��________mL��
��3������300mL 1 mol/LAL2(SO4)3����Һ������Ũ��Ϊ1mol/L��![]() ��Һ��������2.34�˰�ɫ������������NaOH��Һ���������Ϊ__________������
��Һ��������2.34�˰�ɫ������������NaOH��Һ���������Ϊ__________������
��4�����������Ϊ0.1 mol������Mg�۵����ʵ�������Ϊa����100 mL 2 mol��L��1�����ܽ�˻������ټ���460 mL 1 mol��L��1NaOH��Һ�����ó�������Al(OH)3������������a��ȡֵ��Χ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
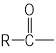
����Ŀ�������ҹ�������ҵ�ķ��ٷ�չ,�Բ��ϵ�����ҲԽ��Խ�࣬�۷���(PAR)�Ƿ��������ϴ������������������ֹ������ϣ���ṹ��ʽΪ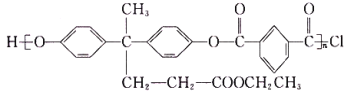 ���ں��պ��������Ӧ�ù㷺����ͼ��������������(CH3COCH2CH2COOH)�ϳɾ۷���E��·��(ʡ�Բ��ֲ���):
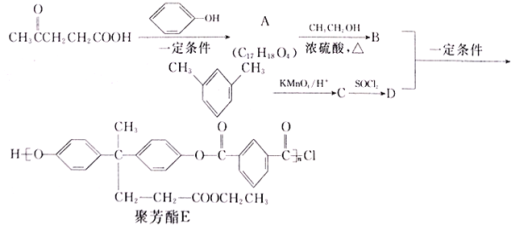
���ں��պ��������Ӧ�ù㷺����ͼ��������������(CH3COCH2CH2COOH)�ϳɾ۷���E��·��(ʡ�Բ��ֲ���):
��֪:  OH+SOCl2--��
OH+SOCl2--�� Cl+SO2 + HCl
Cl+SO2 + HCl
 Cl+R'OH��
Cl+R'OH�� OR'+HCl (R��R' ��ʾ����)
OR'+HCl (R��R' ��ʾ����)
(1)A�к��еĹ�������_____________(�����������)��
(2)A��CH3CH2OH��Ӧ����B�ķ�Ӧ����Ϊ_____________________________��A�Ľṹ��ʽΪ________________________��
(3)д��B+D��E �Ļ�ѧ����ʽΪ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ܱ������У���CO2��H2�����ʵ���֮��Ϊ1:3����Ͷ�ϣ�������Ӧ2CO2(g)+6H2(g)![]() CH3CH2OH(g)+3H2O(g) ��H<0,��5MPa �²�ò�ͬ�¶���ƽ����ϵ�и������ʵ�������� ( V% ) ��ͼ��ʾ������˵������ȷ����
CH3CH2OH(g)+3H2O(g) ��H<0,��5MPa �²�ò�ͬ�¶���ƽ����ϵ�и������ʵ�������� ( V% ) ��ͼ��ʾ������˵������ȷ����
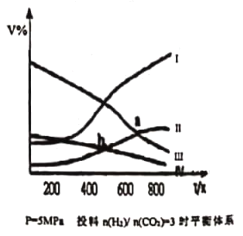
A.��ʾCH3CH2OH��ֵ�������IV
B.ͼ�����߽���a��b ��Ӧ��������Ӧƽ�ⳣ��Ka > Kb
C.ͼ�����߽���a ��Ӧ�� CO2 ת����Ϊ 40%
D.���ס��������ܱ�������ʼʱ���ݻ����¶ȼ�Ͷ�Ϸ�ʽ����ͬ���ף����º�ѹ���ң����º��ݣ���Ӧ��ƽ��ʱCH3CH2OH���ʣ��ף���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����mg Na������������ȼ�գ����ɹ��������Ϊ��m��3.55��g����mg Na��������Ӧ�����ɹ��������Ϊ��������
�٣�m��0.8��g���ڣ�m��1.0��g���ۣ�m��1.2��g���ܣ�m��1.6��g���ݣ�m��1.4��g
A.���٢�B.���٢�C.���ۢ�D.�٢ڢۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڿ��淴Ӧ4NH3(g)��5O2(g) ![]() 4NO(g)��6H2O(g)������������ȷ����(����)
4NO(g)��6H2O(g)������������ȷ����(����)
A. ��ѧƽ�ⳣ���ı���ʽK��![]()
B. ��v��(NH3)��v��(NO)��1��1ʱ��˵���û�ѧ��Ӧ�Ѿ��ﵽƽ��״̬
C. ���ﵽƽ��״̬ʱ���ַ�Ӧ���ת������ȣ�����ʼͶ��ʱn(NH3)��n(O2)��5��4
D. ��Ӧ�ﵽƽ��״̬����������ֻ����NH3��NO�������ɱ���ԭƽ��״̬
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com