
����Ŀ���ζ�ʵ���ǻ�ѧѧ������Ҫ�Ķ���ʵ�飬�������к͵ζ���������ԭ�ζ��ͳ����ζ���
��.����к͵ζ�������֪ijNaOH�����к���NaCl���ʣ��ñ�����ⶨ������NaOH�������������������²���ʵ�飺
�ٳ���һ����������Ʒ����ˮ�������Һ����ȷ��ȡһ�����������Һ����ƿ�У�
�۵μӼ��η�̪��Һ�����ñ�����ζ����ظ��������Σ���ش��������⣺
��1���ζ���ʹ��ʱ�ĵ�һ��������____________________________��
��2������50.00mL�ζ��ܽ���ʵ�飬���ζ����е�Һ���ڿ̶���10����������ڵ����������ţ�_____���٣�10mL���ڣ�40mL����<10mL����>40mL����
��3�����ζ����յ�ʱ���Ӷ��������ɴ˲��ʽ����NaOH�ĺ�����__________������ƫ������ƫС����������������
��.����֪Ũ�ȵĸ��������Һ�ⶨδ֪������Һ��Ũ�ȣ�ȡһ�����δ֪Ũ�ȵIJ�����Һ����ƿ�У���������ϡ���ᣬ����֪Ũ�ȵĸ��������Һ�ζ���
��1��������Ӧ�����ӷ���ʽΪ��__________________________________
��2���ζ�ʱ��KMnO4��ҺӦװ��ͼ��_________���������������������ζ����У��ζ��յ�ʱ�ζ�������________________________________��

��3������������������ⶨ���ƫ�ߵ���_______________________________��
a.װ��Һ�ĵζ���������ˮϴ��δ�ñ�Һ��ϴ
b.������ƿʱ������ƿ����Һ����
c.���ڵζ������в��������α�Һ������ƿ��
d.��ƿ��δ֪Һ��ϴ
e.�ζ�ǰ������ˮ��ϴ��ƿ
���𰸡���© �� ƫС 2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O �� ���������һ��KMnO4����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ acd
��������
��(1)�ζ�����ʹ��ǰ��Ҫ����Ƿ�©Һ��
(2)�ζ��ܵ�0�̶����Ϸ���10mL�̶����·�����40mL�п̶ȵ���Һ������ζ���50mL�̶�������Һ�壻
(3)�ζ����յ�ʱ���Ӷ����������ı�Һ���ƫС���ݴ˷������
��(1)�����������������������ᷢ��������ԭ��Ӧ����Mn2+��CO2��H2O���ݴ���д��Ӧ�ķ���ʽ��
(2)���������Һ����ǿ�����ԣ��ܹ�������ʽ�ζ��ܵ��ܣ��ζ�����ǰ��ҺΪ��ɫ���ζ�����ʱ��Һ��Ϊdz��ɫ���ݴ��жϵζ��յ㣻
(3)���ݲ���������c(����)=![]() ��Ӱ�������
��Ӱ�������
I��(1)�ζ������ܻ���������ʹ��ǰ�����©���ʴ�Ϊ����©��
(2)����50mL�ζ��ܽ���ʵ�飬���ζ����е�Һ���ڿ̶���10�������ζ��ܵ�0�̶����Ϸ���10mL�̶����·�����40mL�п̶ȵ���Һ������ζ���50mL�̶�������Һ�壬��˹��ڵ�Һ�������(50.00mL-10.00mL)=40.00mL���ʢ���ȷ���ʴ�Ϊ���ܣ�
(3)���ζ����յ�ʱ���Ӷ����������ı�Һ���ƫС���������NaOH�����ʵ���ƫС���ɴ˲��ʽ����NaOH�ĺ�����ƫС���ʴ�Ϊ��ƫС��
��(1)�����������ᷴӦ�����ӷ���ʽΪ��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O���ʴ�Ϊ��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O��
(2)��Ϊ��ʽ�ζ��ܣ���Ϊ��ʽ�ζ��ܣ����Ը��������Һ����ǿ�����ԣ�Ӧ������ʽ�ζ���ʢ�ţ������Ը��������Һ�ζ����ᣬ�ζ��յ�����Ϊ�����������һ��KMnO4����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ���ʴ�Ϊ���ף����������һ��KMnO4����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
(3)a��װ��Һ�ĵζ���������ˮϴ��δ�ñ�Һ��ϴ�����±�ҺŨ�ȼ�С���ζ����������ı�Һ���ƫ�ⶨ���ƫ�ߣ���a��ȷ��b��������ƿʱ������ƿ����Һ�������������ı�Һ���ƫС���ⶨ���ƫ�ͣ���b����c�����ڵζ������в��������α�Һ������ƿ�⣬�������ı�Һ���ƫ�ⶨ���ƫ�ߣ���c��ȷ��d����ƿ��δ֪Һ��ϴ�����´���Һ���ƫ�ζ����������ı�Һ���ƫ�ⶨ���ƫ�ߣ���d��ȷ��e���ζ�ǰ������ˮ��ϴ��ƿ����Ӱ���Һ�������Ӱ��ζ��������e���ʴ�Ϊ��acd��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪12.04��1023��O2���ӣ���O2�����ʵ�����________����֪CO2��������88g����CO2�����ʵ�����________����״���£���֪N2�������11.2L����N2�����ʵ�����________����4.0gNaOH��������ˮ�У����2L��Һ�������ʵ����ʵ���Ũ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ���������Ⱦ������Ҫ��ǿ�Թ�ҵ����������β����������������ѧ֪ʶ�ش��������⣺
(1)��ʯȼ�ϰ���ú��ʯ�ͺ�________��
(2)������ָpH____(����>����<������=��)5.6�Ľ�ˮ��ú��ȼ���ǵ��������γɵ���Ҫԭ��������ˮ��pHԼΪ5.6��ԭ����__________________ (�û�ѧ����ʽ��ʾ)��
(3)ú�������Ǹ�Ч���������ú����Ҫ;�����ɽ�ú���ɽ�̿���ٽ���̿�ڸ�������ˮ������Ӧ����һ����̼�������Ļ�ѧ����ʽΪ_________���÷�Ӧ�Ļ���������_________��
(4)������β���ŷſڼ�װ����Ч�������������ڲ������������ʵ�����£��ɽ�β���е�һ����̼��һ������ת��Ϊ�������ѭ����������������壬�÷�Ӧ�Ļ�ѧ����ʽΪ__________________��
(5)������Դ����δ�ռ�ʱ����ͼ��ʾΪһ�������ܼ���������Ч��ʩ�����¶��Ҵ�������ȼ�ϵ������������__________(����ĸ)��
![]()
A.ԭ����Դ�ḻ B.�ǿ�������Դ C.ȼ����ȫû����Ⱦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������У�ǰ�߸պ��Ǻ����������� (����)
A. 2 mol H2O��Ħ��������1 mol H2O��Ħ������
B. 200 mL 1 mol��L-1�Ȼ�����Һ��c(Cl-)��100 mL 2 mol��L-1�Ȼ�����Һ��c(Cl-)
C. 64 g������������ԭ�����ͱ�״����22.4 Lһ����̼����ԭ����
D. 20% NaOH��Һ��NaOH�����ʵ���Ũ�Ⱥ�10% NaOH��Һ��NaOH�����ʵ���Ũ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶��£���Cl2ͨ��NaOH��Һ�У���Ӧ�õ�NaClO��NaClO3�Ļ��Һ�����ⶨClO����ClO3�������ʵ���Ũ��֮��Ϊ1��3����Cl2��NaOH��Һ��Ӧʱ����ԭ����ԭ���뱻��������ԭ�ӵ����ʵ���֮��Ϊ
A.21��5B.11��3C.3��1D.4��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij������Cr2O72������ˮ�����������[FeSO4��(NH4)2SO4��6H2O]��������Ӧ����Ԫ�غ�Ԫ����ȫת��Ϊ�������ó����������õ�nmolFeO��FeyCrxO3�������Ǵ��������е�ʵ����ģ���������������ǣ� ��
A.��FeOFeyCrxO3��3x=y
B.������ˮ��Cr2O72-�����ʵ���Ϊ![]() mol
mol
C.��Ӧ�з���ת�Ƶĵ�����Ϊ3nxmol
D.������������淋����ʵ���Ϊn(2-x)mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ԭ��Ľṹ��ʽ��ͼ�������й���ԭ���˵������ȷ����
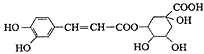
A.����ʽΪC16H18O9
B.����̼������Һ������Ӧ
C.�ܷ���ȡ����Ӧ����ȥ��Ӧ
D.0.1 mol��ԭ������뺬0.6molBr2��Ũ��ˮ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������G��һ��ҩ��ϳ��м��壬��ϳ�·����ͼ��
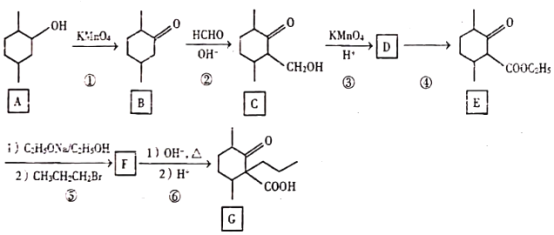
�ش���������
��1��A�еĹ�����������_____��
��2��̼ԭ��������4����ͬ��ԭ�ӻ����ʱ����̼��Ϊ����̼����B�Ľṹ��ʽ�У����Ǻţ�*�����B�е�����̼_____ D�Ľṹ��ʽΪ_____��
��3��������Ԫ���ṹ�����ܷ���������Ӧ��B��ͬ���칹��Ľṹ��ʽ_____�������������칹��ֻ��д��2����
��4����Ӧ��������Լ���������_____��
��5���ݵķ�Ӧ������_____��
��6��д��F��G�ķ�Ӧ����ʽ_____ ������1�Ͳ���2�ֿ�д����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������[(C6H11O7)2Fe]��ҽ���ϳ��õIJ�������������ˮ�������������Ҵ���ijʵ��С��ͬѧ������ͼװ�����Ʊ�FeCO3������FeCO3���������ᷴӦ��һ���Ƶ���������������
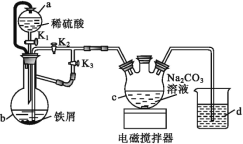
��ش��������⣺
��1������ͨ©���Ƚϣ�a©�����ŵ���___��
��2������ͼ���Ӻ�װ�ã���������Ժ����ҩƷ����K1��K3���ر�K2��
��b�е�ʵ������___��
��һ��ʱ��ر�___����___(ѡ����K1����K2������K3��)���۲쵽b�е���Һ������c�У�ͬʱc������FeCO3������
��b����������������___��
��3����c���Ƶõ�̼�������ڿ����й���ʱ��ϳ�ʱ��������Ϊ���ɫ���û�ѧ����ʽ˵����ԭ��___��
��4��������������̼��������ϣ��뽫��Һ��pH������5.8����ԭ����___����������Һ�м����Ҵ�����������Ʒ�������Ҵ���Ŀ����___��
��5����ͬѧ�����NaHCO3��Һ����Na2CO3��Һ�Ƶõ�̼���������ȸ��ߣ�����ܵ�ԭ����___��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com