
����Ŀ���±���A��B��C��D�����л�����й���Ϣ:
A | ����ʹ������Ȼ�̼��Һ��ɫ���ڱ���ģ��Ϊ�� |
B | ����C��H����Ԫ����ɣ������ģ��Ϊ |
C | ����C��H��O����Ԫ����ɣ�������Na��Ӧ����������NaOH��Һ��Ӧ��������D��Ӧ������Է�������Ϊ100���� |
D | ����C��H��O����Ԫ����ɣ������ģ��Ϊ |
�ش���������:
��1��A��������Ȼ�̼��Һ��Ӧ��������Ĺ���������Ϊ________��д��A��һ�������·�Ӧ���ɸ߷��ӻ�����ķ���ʽ��___________________��
��2��A�����������ӳɷ�Ӧ����������E,��E�ڷ�����ɺͽṹ�����Ƶ��л�����һ����(�׳���ͬϵ����),���Ǿ�����ͨʽCnH2n+2����n=___________________ʱ�������л��↑ʼ����ͬ���칹�塣
��3������B��˵����ȷ���ǣ�___________________��
a������ԭ����ͬһ��ƽ���� b������̼ԭ��֮���ǵ�˫������Ľṹ
c��һ��B���Ӻ���6������ d�����б����Ļ������Ϊ���㻯����
��4��C��D��Ӧ��������Է�������Ϊ100����,�÷�Ӧ�ķ�Ӧ����Ϊ___________________;�仯ѧ����ʽΪ___________________��
���𰸡���ԭ�� nCH2=CH2![]()
![]() 4 ad ������Ӧ��ȡ����Ӧ�� CH2=CH��COOH+C2H5OH
4 ad ������Ӧ��ȡ����Ӧ�� CH2=CH��COOH+C2H5OH![]() CH2=CH��COOC2H5+H2O
CH2=CH��COOC2H5+H2O
��������
A��ʹ������Ȼ�̼��Һ��ɫ�����A�ı���ģ�ͣ�A�Ľṹ��ʽΪCH2=CH2��A��ˮ��Ӧ���ɵ�CΪCH3CH2OH��B��������������ģ�ͣ�B�Ľṹ��ʽΪ![]() ��DΪ���ĺ��������D��C��Ӧ������Է�������Ϊ100������������ģ�ͣ�D�Ľṹ��ʽΪCH2=CHCOOH��
��DΪ���ĺ��������D��C��Ӧ������Է�������Ϊ100������������ģ�ͣ�D�Ľṹ��ʽΪCH2=CHCOOH��
��1��A��������Ȼ�̼��Һ��Ӧ���������飬�������й����ŵ���������ԭ�ӣ���ϩ��һ�������·�Ӧ���ɾ���ϩ�ķ���ʽΪnCH2=CH2![]()
![]() ��
��
��2����ϩ�������ӳ��������飬������������������̼�����ڵ���4ʱ��������ʼ����ͬ���칹�壬��Ϊ����̼�����ڵ���4ʱ����ʼ���ֲ�����
��3����Ϊƽ���ͷ��ӣ��ʱ�������ԭ�Ӿ���ͬһ��ƽ���ϣ�a��ȷ�������в�����˫���������е�̼̼���ǽ��ڵ�����˫��֮���һ������ļ���b��������̼̼֮�����6��������̼��֮�����6����������һ������һ������12��������c�����ݷ����廯����Ķ��壬���ڱ������л�����Ƿ����廯���d��ȷ����Ϊad��
��4��CΪ����DΪ�ᣬ���߿��Է���������Ӧ����Ӧ����ʽΪCH2=CH��COOH+C2H5OH![]() CH2=CH��COOC2H5+H2O��
CH2=CH��COOC2H5+H2O��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)����C5H12��һ��ͬ���칹��ֻ������һ��һ�ȴ�����칹��Ľṹ��ʽΪ______��
(2)2��2��3��3-�ļ�����Ľṹ��ʽΪ��___________��
(3)![]() ��ϵͳ������________��
��ϵͳ������________��
(4)1mol�����������Ժ�1molCl2�����ӳɷ�Ӧ������2-��-2,3-�������飬��ò��������Ľṹ��ʽ�ǣ�______��
(5)��Է���������Ĵ���������ȣ����ķе�ԶԶ����������ԭ����__________��
(6)��ͬ�����ļ��飬���飬��ϩ����Ȳ����ȫȼ�պ�����CO2������_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵ�����A��B�����2L���ܱ������У��������·�Ӧ��3A(g)+B(g)xC(g)+2D(g)����6min����D��Ũ��Ϊ0.75mol/L��c(A):c(B)=3:5����C��ʾ��ƽ����Ӧ����v(C)=0.125mol/(L��min)������˵����ȷ����
A.��B��ʾ��ƽ����Ӧ����Ϊv(B)=0.03125mol/(L��min)
B.6minʱ��ת���˵�Aռ��ʼA��50��
C.�÷�Ӧ����ʽ�У�x=3
D.6minʱ��A�����ʵ���Ϊ0.75mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��ԭ��������������Ķ�����Ԫ�أ�A�Ƕ������н�������ǿ��Ԫ�أ�B�ǵؿ��к�����ߵĽ���Ԫ�أ�C���ʵľ��������õİ뵼����ϣ�D���������������ڲ������֮��Ϊ3:5��
(1)D��Ԫ�����ڱ��е�λ��______��
(2)A������������ȼ�����ɻ�����ף�����������ѧ������Ϊ______��A������E������ȼ�����ɻ������ң��õ���ʽ��ʾ�ҵ��γɹ���______��
(3)F����ѧ��ѧ����Ԫ�أ�����һ��������Ϊ����ɫ��ĩ��B���������ڸ��������·�Ӧ��ұ��F���ʵķ���֮һ���÷�Ӧ�Ļ�ѧ����ʽΪ______��
(4)A��B��D��E�����Ӱ뾶�ɴ�С��˳��Ϊ��______��(���û�ѧ������д����ͬ)��C��D��E����̬�⻯����ȶ���˳��Ϊ:______��
(5)��ҵ�Ͻ������E����ͨ��D���ڵĵ����п��Ƶû�����D2E2�������ʿ���ˮ��Ӧ����һ����ʹƷ����Һ��ɫ�����壬0.2mol�����ʲμӷ�Ӧʱת��0.3mol���ӣ�����ֻ��һ��Ԫ�ػ��ϼ۷����ı䣬�÷�Ӧ�Ļ�ѧ����ʽΪ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������Ǻ���һ�����һ�������ˮƽ����Ҫ��־����������������У���ؼ���һ����ӦΪ��2SO2(g)+O2(g)![]() 2SO3(g)��
2SO3(g)��
��1��һ�������£�SO2��O2��Ӧ10min����SO2��SO3���ʵ���Ũ�ȷֱ�Ϊ1mol/L��3mol/L����SO2��ʼ���ʵ���Ũ��Ϊ___________________��10min����SO3�Ļ�ѧ��Ӧ����Ϊ___________________��
��2�����й��ڸ÷�Ӧ��˵����ȷ����___________________��
A������O2��Ũ���ܼӿ췴Ӧ����
B��������ϵ�¶��ܼӿ췴Ӧ����
C��ʹ�ô����ܼӿ췴Ӧ����
D��һ�������´ﵽ��Ӧ��ʱSO2ȫ��ת��ΪSO3
��3����ҵ�����ᣬ�ù����İ�ˮ��SO2β����������д����ص����ӷ���ʽ:____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڷ��ȷ�ӦH2 + Cl2 ![]() 2HCl����˵������ȷ����
2HCl����˵������ȷ����
A. �÷�Ӧ��,��ѧ��ֻת��Ϊ����
B. ��Ӧ�������е����������ڲ��������е�������
C. ���������е����������ڷ�Ӧ�������е�������
D. �Ͽ�1mol H-H����1mol Cl-Cl�������յ�������С���γ�2mol H-Cl�����ų�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��G�����л���������ǵ�ת����ϵ���£�
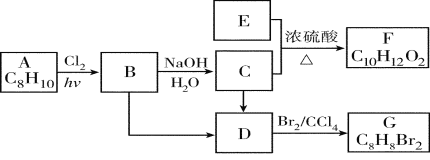
��ش��������⣺
(1)��֪��6.0 g������E��ȫȼ������8.8 g CO2��3.6 g H2O��E������������������ܶ�Ϊ30����E�ķ���ʽΪ________��
(2)AΪһȡ��������B�к���һ��������B����C�Ļ�ѧ����ʽΪ��________________��
(3)��B����D�ķ�Ӧ������________________��
(4)��G��ͬ���칹���У�������һ�����IJ���ֻ��һ�ֵĹ���________�������к˴Ź�������������壬�ҷ������Ϊ1��1����_________(��ṹ��ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����пͭԭ��س��У���ͼ
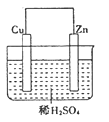
(1)пΪ______����������______��Ӧ���缫��Ӧʽ_________________________________���۲쵽��������_______________��
(2)ͭΪ______����������______��Ӧ���缫��Ӧʽ ______________���۲쵽��������__��
(3)����������_______________����
(4)���Ӵ� ______________���� _______________����
(5)����·��ת��0.2mol���ӣ����������������__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͭ����Ҫ�Ĺ�ҵԭ�ϣ�����![]() ��
��![]() ��ɵĻ���ij�о���ѧϰС��Ϊ��̽������������ȡ
��ɵĻ���ij�о���ѧϰС��Ϊ��̽������������ȡ![]() �û�������
�û�������![]() ��ϡ�����У�����������ȫ��Ӧ������
��ϡ�����У�����������ȫ��Ӧ������![]() (��״��)��
(��״��)��![]() ����������Һ�м���
����������Һ�м���![]() ��
��![]() ��Һ��ǡ��ʹ��Һ�е�
��Һ��ǡ��ʹ��Һ�е�![]() ������ȫ��
������ȫ��
(1)![]() ��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ____________��
��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ____________��
(2)�������![]() ______��
______��![]() ________��
________��
(3)![]() ______
______
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com