
����Ŀ��ij�������ؾ�ʯ����Ҫ��BaSO4��Ϊԭ�ϣ������������մɹ�ҵ֧������������(BaTiO3)�Ĺ����������£�
�ش��������⣺
(l)Ϊ���BaCO3��������ʣ��ɲ�ȡ�Ĵ�ʩΪ__��д��һ�����ɣ��������£�TiCl4ΪҺ������ˮ�⣬����һ��Ũ�ȵ�TiCl4��Һ�ķ����� ____��
(2)��Na2CO3��Һ�����ؾ�ʯ���������ʲ���Na2CO3��Ӧ�����ܽ�BaSO4ת��ΪBaCO3���˷�Ӧ��ƽ�ⳣ��K= ___����д������������������CO32����ˮ�⣬Ҫʹ2. 33g BaSO4ǡ����ȫת��ΪBaCO3����������ҪŨ��Ϊ1.0mol��L��1Na2CO3��Һ ___mL������֪��Ksp(BaSO4)=1.0��10-10��Ksp(BaCO3)=5.0��10-9��
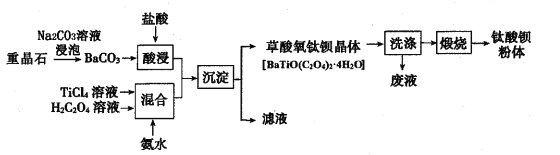
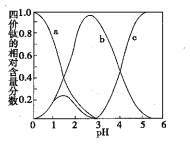
(3)���������������Һ����Ԫ���ڲ�ͬpHʱ��Ҫ��TiO(OH)����TiOC2O4��TiO(C2O4)22��������ʽ���ڣ��仯��������ͼ��ʾ����ʵ���Ʊ������У����ð�ˮ���ڻ����Һ��pH��2.8���ң��ٽ��������������䷴Ӧ�����ӷ���ʽΪ____��ͼ������c��Ӧ�ѵ���ʽΪ____�������ӷ��ţ���
(4)����������Һ������Ҫ�ɷ�Ϊ____�������������ղ������ѱ�����õ����ᱵ�������̬�����д����Ӧ�Ļ�ѧ����ʽ��____��
���𰸡���BaCO3�гɷ�ĩ���ʵ���������Ũ�Ȼ��ʵ����Ȼ����� ����Ũ���ᣬ�ټ�����ˮϡ��������Ũ�� 0.02 510 TiO(C2O4)22����Ba2����4H2O=BaTiO(C2O4)2��4H2O�� TiO(OH)�� NH4Cl BaTiO(C2O4)2��4H2O![]() BaTiO3��2CO����2CO2����4H2O
BaTiO3��2CO����2CO2����4H2O
��������
(1)�������ʵĴ���״̬��Ӱ�컯ѧ��Ӧ���ʵ����ط�����TiCl4��ǿ�������Σ�����Һ������������ˮ�⣬��������ˮ�⿼�ǣ�
(2)���ݳ�����ת��ƽ�ⷽ��ʽ��д�����㣻
(3)�������Һ�������У���Ԫ���ڲ�ͬpH����Ҫ��TiOC2O4��TiO(C2O4)22-��TiO(OH)+������ʽ���ڣ����ڻ��ҺpH��2.8�����ٽ��г������ݡ�������ʱ���ɲ������ѱ������к���TiO(C2O4)22-(��b��)���ٸ��ݷ�Ӧǰ�����Ԫ�ص�ԭ�Ӹ����غ㼰����Ļ�ѧʽ��д����Ӧ�Ļ�ѧ����ʽ�����Ű�ˮ�IJ��ϼ�����Һ��pH�����������ж�c��Ӧ�ѵ���ʽΪTiO��OH��+��
(4)����ԭ���غ��ж���Һ�е���Ҫ�ɷ֣��������ѱ���������������յõ�BaTiO3��ͬʱ�õ�CO��CO2��ˮ������
(1)������BaCO3������Ӧ��2HCl+BaCO3=BaCl2+H2O+CO2����Ϊ�˼ӿ췴Ӧ���ʿ��Խ�����BaCO3�гɷ�ĩ��������Ӵ������Ҳ�����ʵ���������Ũ�Ȼ��ʵ��������߷�Ӧ�¶Ȼ����ȣ�TiCl4��ǿ�������Σ�����Һ��Ti4+�ᷢ��ˮ�ⷴӦ��Ti4++4H2O![]() Ti(OH)4+4H+��ʹ��Һ����ǣ�Ϊ�����Ƶõ������TiCl4��Һ��ͬʱ�������������ӣ�ͨ���ǽ�TiCl4����Ũ�����У�Ȼ���ټ�����ˮϡ��������Ũ�ȣ�
Ti(OH)4+4H+��ʹ��Һ����ǣ�Ϊ�����Ƶõ������TiCl4��Һ��ͬʱ�������������ӣ�ͨ���ǽ�TiCl4����Ũ�����У�Ȼ���ټ�����ˮϡ��������Ũ�ȣ�
(2) ����Һ��BaSO4���ڳ����ܽ�ƽ�⣬������Һ�м��뱥��Na2CO3��Һʱ����������ת������BaCO3��BaSO4(s)+CO32-(aq)![]() BaCO3(s)+SO42-(aq)�����ﵽƽ��������ϲ���Һ���ظ���β���������BaSO4����BaCO3����Ӧ��ƽ�ⳣ��
BaCO3(s)+SO42-(aq)�����ﵽƽ��������ϲ���Һ���ظ���β���������BaSO4����BaCO3����Ӧ��ƽ�ⳣ��![]() ��2.33gBaSO4���ʵ���Ϊ0.01mol������BaSO4(s)+CO32-(aq)
��2.33gBaSO4���ʵ���Ϊ0.01mol������BaSO4(s)+CO32-(aq)![]() BaCO3(s)+SO42-(aq)����ȫת����Ҫ0.01molCO32����ͬʱ��Һ�в���0.01molSO42-������Kֵ��Һ�к���n��CO32-��=0.01mol��0.02=0.5mol����Ҫ����Na2CO3Ϊ0.01mol+0.5mol=0.51mol����ҪNa2CO3��Һ�����Ϊ0.51mol��1mol/L=0.51L=510mL��
BaCO3(s)+SO42-(aq)����ȫת����Ҫ0.01molCO32����ͬʱ��Һ�в���0.01molSO42-������Kֵ��Һ�к���n��CO32-��=0.01mol��0.02=0.5mol����Ҫ����Na2CO3Ϊ0.01mol+0.5mol=0.51mol����ҪNa2CO3��Һ�����Ϊ0.51mol��1mol/L=0.51L=510mL��
(3) �������Һ�������У���Ԫ���ڲ�ͬpH����Ҫ��TiOC2O4��TiO(C2O4)22-��TiO(OH)+������ʽ���ڣ����ڻ��ҺpH��2.8�����ٽ��г������ݡ�������ʱ���ɲ������ѱ������к���TiO(C2O4)22-(��b��)����������ʱ�����ӷ���ʽΪ��TiO(C2O4)22-��Ba2��+4H2O=BaTiO(C2O4)2��4H2O�������Ű�ˮ�IJ��ϼ�����Һ��pH�����������ж�c��Ӧ�ѵ���ʽΪTiO��OH��+��
(4) TiCl4�Ͳ�����Һ�����˰�ˮ��Ȼ����BaCl2��Һ��ϵõ��������ѱ����壬����ԭ���غ㣬��Һ����Ҫ�ɷ�ΪNH4Cl���������ѱ���������������յõ�BaTiO3��ͬʱ�õ�CO��CO2��ˮ���������ղ������ѱ�����õ�BaTiO3����ʽΪBaTiO(C2O4)2��4H2O ![]() BaTiO3+2CO��+2CO2��+4H2O��
BaTiO3+2CO��+2CO2��+4H2O��


 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���¶��µ�ij�����ܱ������У��������л�ѧƽ�⣺C(s)+H2O(g) ![]() CO(g)+H2(g)�����϶��������淴Ӧ��һ���������Ѵﵽ��ѧƽ��״̬����
CO(g)+H2(g)�����϶��������淴Ӧ��һ���������Ѵﵽ��ѧƽ��״̬����
A.��ϵѹǿ���ٷ����仯B.v��(CO)=v��(H2O)
C.���� n molCO ��ͬʱ���� n mol H2D.1 mol HH �����ѵ�ͬʱ�γ� 2 mol HO ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ŀǰ�ҹ���Ҫ�������ᷨ�����Ѱף�TiO2����ÿ���������100����Ѱ�Һ�����к�H2SO4Լ20%����������Fe2+��TiO2+��Al3+�������Ѱ�Һ��������п������ˮ����п������ԭ���á����ռ������ʸߺͲ�Ʒ���ȸߵ��ŵ㡣�������̼����£�

�ش��������⣺
��1����������ʱ��������Ҫ��Ӧ�����ӷ���ʽΪ_______��
��2��ΪѰ�ҡ��������������������9��Ա�ʵ����±���
���� | �¶�/�� | ��Ӧʱ��/h | ��Һ�� | ����/% |
1 | 70 | 2 | 1��6.5 | 79.83 |
2 | 70 | 3 | 1��7.5 | 86.18 |
3 | 70 | 4 | 1��8.5 | 84.33 |
4 | 80 | 2 | 1��7.5 | 83.06 |
5 | 80 | 3 | 1��8.5 | 87.02 |
6 | 80 | 4 | 1��6.5 | 95.38 |
7 | 90 | 2 | 1��8.5 | 83.58 |
8 | 90 | 3 | 1��6.5 | 88.95 |
9 | 90 | 4 | 1��7.5 | 89.64 |
�ɱ������ݿ�֪���������ʡ����ʱ�ķ�Ӧ������_______��
��3��������ʵ�ʿ��ǣ�����Һ��pHʱѡ�����ʯ�����ԭ����_______��
��4�������£���1:6��Һ�����ȷ�Ӧ�Ľ���Һ��п����Ũ�����ֵԼΪ2.5mol/L����Ksp[Zn(OH)2]��1.2��10-17��lg4.8��0.68�������ʯ������ڵ�pHӦ������______������һλС������
��5���ڼ������������ʱ��������Ӧ�Ļ�ѧ����ʽ��___����ʱTiO2+Ҳ��ˮ������H2TiO3������������Ӧ�����ӷ���ʽΪ_____��
��6�������¼���H2O2Ŀ���ǽ�һ���������ӣ���������Ϊ__�����˵õ���������Ҫ�ɷ���___��H2TiO3���ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ����0.1mol��L-1NaA��Һ����εμ����ᣬ��û����Һ��pH��p![]() �仯��ϵ��ͼ��ʾ[
�仯��ϵ��ͼ��ʾ[![]() =-lg
=-lg![]() ]������˵����ȷ����
]������˵����ȷ����

A. a����Һ��c��Na+��=c��A-��
B. ���볣��K��HA����������Ϊ10-4
C. �μӹ�����![]() ���ϼ�С
���ϼ�С
D. b����Һ��c��HA����c��Na+����c��A-����c��OH-��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ⷨ���Ѱ۲����ķ�Һ[���д�����FeSO4��H2SO4��������Fe2��SO4��3��TiOSO4]��������Ͳ�Ѫ�����������Ĺ���������ͼ��ʾ��

��֪:TiOSO4������ˮ����ˮ�п��Ե���ΪTiO2+��SO42-��TiO2+ˮ���TiO2��xH2O����Ϊ���淴Ӧ������ṹ��ʽΪCH3CH��OH��COOH��
�ش��������⣺
��1��TiOSO4����Ԫ�صĻ��ϼ���____________��������з�������������Һ�������IJ�����___________��
��2����������Ҫ�ɷ�ΪTiO2��xH2O��������ӷ���ʽ���͵õ�������ԭ��________��
��3��������������Һ�еõ�������������IJ���������____________________�����������ڿ�������������������������÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ______��
��4��������з�����Ӧ�����ӷ���ʽΪ______________________��
��5������ޱ������һ������նȣ�ԭ��������������ˮ�Լ�____________________��
��6��ʵ�����м�����ҺB����Ҫ�����ӵķ�����______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������°�V�����������1���ˮ�еõ����ı�����Һ�������������ա�
(1)NH3ͨ��ˮ�з����ķ�ӦΪ____________________��
(2)��ˮ�д��ڵķ�����__________________________��
(3)��ˮ�д��ڵ�������__________________________��
(4)���ð�ˮ����������Ϊ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���42��XԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӣ�YԪ��ԭ�ӵ������2p�������2��δ�ɶԵ��ӡ�X��Y���γɻ�����X2Y3��ZԪ�ؿ����γɸ�һ�����ӡ���ش��������⣺
��1��XԪ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ__________����Ԫ�صķ�����__________;
 ��2��YԪ��ԭ�ӵļ۲���ӵĹ����ʾʽΪ________����Ԫ�ص�������__________;
��2��YԪ��ԭ�ӵļ۲���ӵĹ����ʾʽΪ________����Ԫ�ص�������__________;
��3��X��Z���γɻ�����XZ3���û�����Ŀռ乹��Ϊ____________;
��4����֪������X2Y3��ϡ������Һ�пɱ�����п��ԭΪXZ3�����ﻹ��ZnSO4��H2O���÷�Ӧ�Ļ�ѧ����ʽ��_________________________________________________;
��5���Ƚ�X���⻯����ͬ��ڶ�����������Ԫ�����γɵ��⻯���ȶ��ԡ��е�ߵͲ�˵������____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£���ij�ܱ������з�����Ӧ��2HI(g)![]() H2(g)��I2(g)����H>0����15s��c(HI)��0.1mol��L��1����0.07mol��L��1��������˵����ȷ����(��)
H2(g)��I2(g)����H>0����15s��c(HI)��0.1mol��L��1����0.07mol��L��1��������˵����ȷ����(��)
A. 0��15 s����I2��ʾ��ƽ����Ӧ����Ϊv(I2)��0.002 mol��L��1��s��1
B. c(HI)��0.07 mol��L��1����0.05 mol��L��1����ķ�Ӧʱ��С��10 s
C. �����¶�����Ӧ���ʼӿ죬�淴Ӧ���ʼ���
D. ��С��Ӧ��ϵ���������ѧ��Ӧ���ʼӿ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ũҵ�����벻�����ʣ���ѧ����ʹ�õ��ʣ�����������ʵ�ʹ���ʣ������ܹ����õر�����������ش��������⣺
��1����N2ת��Ϊ����������Ĺ��̳�Ϊ�̵���
���˹��̵�����N2��NH3��N2����___��Ӧ�����������ԭ����
����Ȼ��̵����ɽ�����N2ת��ΪCa��NO3��2�ȵ��ʣ�ת��;�����£�ת�������Լ�����������ȥ����N2��NO��NO2��HNO3![]() Ca��NO3��2��
Ca��NO3��2��
д��NO��NO2��HNO3�Ļ�ѧ����ʽ___��___��
��HNO3ת��ΪCa��NO3��2���о����ֲ�ͬ���Ļ�����M___��д��ѧʽ����
��2����ѧ�����ر��桢ʩ�õ��ʡ�
��NH4HCO3�����������棬ԭ����___��д��ѧ����ʽ����
���̬���ʲ�������Է��ϻ��ʹ�ã���NH4ClΪ��д��������Ӧ�����ӷ���ʽ___��
��3������ʩ�õ��ʽ����´�����NH3�������ߣ��Ӿ��������γɡ���NH4��2SO4�������ijɷ�֮һ�����γɹ�����ͼ��ʾ��ת�������Լ�����������ȥ����

��Y��NH3��Ӧ���ɣ�NH4��2SO4�Ļ�ѧ����ʽ___��
������CO��NH2��2��һ�ֳ��û��ʣ�������H2O������������ԭ��Ӧ�ͷų�NH3����������CԪ�ػ��ϼ�Ϊ___��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com