
����Ŀ��ʯ���ǹ�ҵ��ѪҺ�������ǵ�����������ϢϢ��ء�
��1����ϩ��ʯ�ͻ�����Ҫ�Ļ���ԭ�ϡ� д����ϩ�ĵ���ʽ____________
��2������ʽΪC5H12��ij���������к���4�����������Ľṹ��ʽΪ_______________________��
��3������ϩ��Ϊͬϵ�����_______ ��(ѡ����)
a. CH3CH=CH2 b. CH2=CHCH=CH2
c. CH��CH d. CH3CH3
��4������ϩ��ȫ������������ʳƷ��װ��������ϩ�Ľṹ��ʽΪ ________________ ��
��5����Ȳ���ۿɵõ��������ϩ����Ȳ (CH2=CH-C-C-CH=CH2) �����𱽺Ͷ���ϩ����Ȳ���õ��Լ���__________________��
���𰸡��� 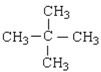 a
a ![]() ��ˮ�����Ը��������Һ
��ˮ�����Ը��������Һ
��������
������ϩΪ���ۻ������Cԭ�Ӻ�Hԭ�������ɴﵽ�ĵ�����д����ϩ�ĵ���ʽ��
�������ķ���ʽ�ͺ����ĸ���д���ṹ��ʽ��
����ͬϵ���������ƣ�����ʽ���n��CH2�����ѡ�������ʵĹ������жϣ�
���ݾ���ϩ������ϩͨ���Ӿ۵õ���д���ṹ��ʽ��
�ҳ��뱽����Ӧ�������ϩ����Ȳ��Ӧ������ɫ�����ʼ��ɼ�����������ʡ�
��1����ϩΪ���ۻ����Cԭ�������ɴﵽ8�����ӣ�Hԭ�������ɴﵽ2�����ӣ���ϩ��ȷ�ĵ���ʽΪ��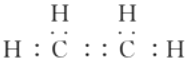 ��
��
��2���������ķ���ʽ�ͺ����ĸ�����֪������ֻ��Ϊ�����飬�ṹ��ʽΪ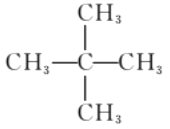 ��
��
��3������ͬϵ��Ķ����֪����ϩ��Ϊͬϵ���ֻ����a��
��4������ϩΪ��ϩͨ���Ӿ۷�Ӧ���ɵģ�����ȷ�Ľṹ��ʽΪ��![]() ��
��
��5�����ݱ�������ˮ������������Һ����Ӧ������ϩ����Ȳ������ˮ������������Һ����Ӧ��ʹ��ˮ������������Һ����ɫ��֪������ˮ������������Һ�����𱽺Ͷ���ϩ����Ȳ�����н���ˮ������������Һ�����뵽����ʱ���ܹ۲쵽����������Һ�ֲ㣬�������ϣ�ˮ���£������ú���ʺ���ɫ��ˮ����ɫ��dz������Һ�ֲ㣬�������ϣ�ˮ���£������ú�����ɫ��ˮ���Գ���ɫ����


 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ����
A.�ھƾ��Ƽ��������£�Na2CO3��NaHCO3���嶼�ܷ����ֽ�
B.Cl2��һ���ж����壬������������ˮ��ɱ������
C.SiO2���ܺ�NaOH��Һ��Ӧ���ܺ�����ᷴӦ������������������
D.Na2SiO3ˮ��Һ�׳�ˮ���������Ʊ��轺��ľ�ķ�����ȵ�ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��Ӧ��2SO2(g)��O2(g)![]() 2SO3(g)��H<0��ij�¶��£��� 2 mol SO2 �� 1 mol O2 ���� 10L �ܱ������У���Ӧ��ƽ���SO2 ��ƽ��ת����(��)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ����ʾ��������˵����ȷ���ǣ� ��
2SO3(g)��H<0��ij�¶��£��� 2 mol SO2 �� 1 mol O2 ���� 10L �ܱ������У���Ӧ��ƽ���SO2 ��ƽ��ת����(��)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ����ʾ��������˵����ȷ���ǣ� ��
�� ��
�� ��
��
A.��ͼ���ƶϣ�B �� SO3��ƽ��Ũ��Ϊ 0.3molL1
B.��ͼ���У��ڴ��¶��£�C �� �� ������ ��
C.�ﵽƽ�����������䣬���뺤����ѹǿ������Ӧ���ʱ仯ͼ�������ͼ�ұ�ʾ
D.ѹǿΪ 0.50 MPa ʱ����ͬ�¶��� SO2 ��ƽ��ת������ʱ���ϵ��ͼ������ T2>T1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ������������ء�
I.K2Cr2O7�����ڼ��˾���Ƿ�ƺ��ʻ��Cr2O72-(��ɫ)+CH3CH2OH��Cr3+(��ɫ)+CH3COOH(δ��ƽ��
��1����̬Crԭ�ӵļ۵��ӹ������ʽΪ__��
��2��CH3COOH����������Ԫ�صĵ縺���ɴ�С��˳��Ϊ__��̼ԭ�ӵĹ���ӻ�����Ϊ__��������������������Ŀ֮��Ϊ__��
��3����֪Cr3+�ȹ���Ԫ��ˮ�����ӵ���ɫ�����ʾ��
���� | Sc3+ | Cr3+ | Fe2+ | Zn2+ |
ˮ�����ӵ���ɫ | ��ɫ | ��ɫ | dz��ɫ | ��ɫ |
�����ԭ�ӽṹ�Ʋ�Sc3+��Zn2+��ˮ������Ϊ��ɫ��ԭ��Ϊ__��
II.ZnCl2Ũ��Һ�����ڳ�ȥ��������������������FeO��Ӧ�ɵ�Fe[Zn(OH)Cl2]2��Һ��
��4��Fe[Zn(OH)Cl2]2��Һ�в����ڵ�������������__(��ѡ����ĸ)��
A.���Ӽ� B.���ۼ� C.������ D.��λ�� E.���»��� F.���
��Һ��[Zn(OH)Cl2]-�ĽṹʽΪ__��
III.п������������Ԫ��֮һ����ѻ���ʽ��ͼ1�������ṹ��ͼ2��
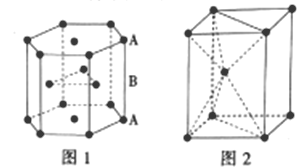
��5��п�Ķѻ���ʽΪ__����λ��Ϊ__��
��6����пԭ�ӵİ뾶Ϊapm�������ӵ�������ֵΪNA����п������ܶ�Ϊ___g/cm3(�ú�a�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ͻ����������ʵ��������������������һ����
��� | ʵ����������� | ���ͻ���� |
A | Ũ����ε�ֽ���ϣ�ֽ��� | Ũ��������ˮ�� |
B | ����ɫʯ����Һ�м�����ˮ����Һ�ȱ�죬�����ɫ | ��ˮ�к����������ʺ� Ư�������� |
C | ��ij��Һ�м���ϡ���ᣬ������ʹ����ʯ��ˮ����ǵ����� | ����Һ��һ����CO32- |
D | ��ij��Һ�м���ŨNaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������� | ����Һ��һ������NH |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��д��ȷ����
A. �����ܽ�ˮ���е�CaCO3�� CaCO3 + 2H+= Ca2++ H2O + CO2��
B. ���Ե缫��ⱥ��MgCl2��Һ�� Mg2++2Cl�� + 2H2O ![]() Mg(OH)2�� + H2�� + Cl2��
Mg(OH)2�� + H2�� + Cl2��
C. ��������Һ��ͨ��������CO2��![]() +H2O+CO2��
+H2O+CO2�� +
+![]()
D. ��������Һ������ȩ�е�ȩ����CH3CHO��![]() ��2OH��
��2OH��![]() CH3COONH4��H2O��2Ag����3NH3��
CH3COONH4��H2O��2Ag����3NH3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ȫ��˳������ʵ�鼰�����˺��ı���.����ʵ�������ȷ�Ҳ��Ǵ�ʵ�鰲ȫ�Ƕȿ��ǵ��ǣ� ��

A. ��������ʹ���Խ���Һ���µĵ���©�����������Ĵ���
B. ��������ʹ��CCl4��ȡ��ˮ�е���ʱ���������ʹ©��������ų�
C. �����������հ������Ȼ������岢��ֹ����
D. ����������ʳָ��סƿ������һֻ����סƿ�ף���ƿ�������������ƿ�Ƿ�©ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���G�Ǻϳ���ũҩ����Ҫ�м��塣�Ի�����AΪԭ�Ϻϳɻ�����G�Ĺ����������£�

��1��������G�к��������ŵ�����Ϊ________��
��2����ӦD��E�ķ�Ӧ����Ϊ________��
��3��������B�ķ���ʽΪC7H6Cl2��B�Ľṹ��ʽΪ______��
��4��д��ͬʱ��������������G��һ��ͬ���칹��Ľṹ��ʽ��______��
���ܷ���������Ӧ��
�ں˴Ź���������ʾ��ԭ�ӵķ�ֵ��Ϊ3��2��2��1��
��5�����Ի�����F��CH2(COOC2H5)2Ϊԭ���Ʊ� ��д���Ʊ��ĺϳ�·������ͼ�����Լ����ã��ϳ�·������ͼʾ����������ɣ���
��д���Ʊ��ĺϳ�·������ͼ�����Լ����ã��ϳ�·������ͼʾ����������ɣ���
__________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʳ�ף���Ҫ�ɷ�CH3COOH �������Na2CO3 ����С�մ�NaHCO3 ����Ϊ��ͥ�����г��õ����ʡ���֪��CH3COOH��H2CO3��HNO2�ĵ��볣����25�棩�ֱ�ΪKa=1.8��10-5��Ka1=4.3��10-7��Ka2=5.6��10-11��Ka=5.0��10-4��ش��������⣺
��һ���¶��£���0.1mol/LCH3COOH ��Һ�м�������CH3COONa ����ʱ������˵����ȷ����____������š���
a����Һ��pH���� b��CH3COOH�ĵ���̶�����
c����Һ�ĵ����������� d����Һ��c(OH-)��c(H+)����
��25��ʱ����CH3COOH��Һ�м���һ������NaHCO3�����û��Һ��pH=6������Һ��:
c(CH3COO-)/c(CH3COOH)=____
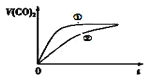
�dz����£���20mL 0.1mol/L CH3COOH��Һ ��20mL 0.1mol/LHNO2 ��Һ�ֱ��� 20mL 0.1mol/LNaHCO3��Һ��ϣ�ʵ���ò��������������V����ʱ�䣨t���ı仯��ͼ��ʾ�����ʾCH3COOH��Һ��������_______����д��ţ���
�������Ϊ100mL pH=2��CH3COOH��һԪ��HX����ˮϡ������pH����Һ����Ĺ�ϵ��ͼ��ʾ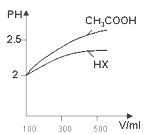 ����HX�ĵ���ƽ�ⳣ��______����������������С����������������CH3COOH�ĵ���ƽ�ⳣ����
����HX�ĵ���ƽ�ⳣ��______����������������С����������������CH3COOH�ĵ���ƽ�ⳣ����
��25��ʱ����������������ʵ���Ũ�ȵĴ����백ˮ��Ϻ���Һ��pH=7����NH3��H2O�ĵ��볣��Kb =___________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com