
����Ŀ�������仯������������������������Ҫ����;����֪: Sn���۵�Ϊ231����Sn2+��ˮ�⡢�ױ�������SnCl4����ˮ�⡢�۵�Ϊ-33�桢�е�Ϊ114�档��ش���������:
��1��Ԫ������ͬ����̼����������3,����ԭ������Ϊ_________��
��2�����������������������۴��Ȳⶨ(��֪�������ԭ������Ϊ119): ��ȡ1.19g��������ϡ������(���ʲ����뷴Ӧ),ʹSn ��ȫת��ΪSn2+���ڼ��������Fe2(SO4)3������0.1000mol/LK2Cr2O7��Һ�ζ�(������Cr��+3��),����20.00mL��������м���Fe2(SO4)3��������________����������Ʒ��������������Ϊ_________�����ڵζ��յ�ʱ���Ӷ�������ᵼ�²�õ���Ʒ��������������________(����ƫ����ƫ����������Ӱ����)��
��2�����ڶ�����ҵ����������(SnSO4)���Ʊ�·������:

�ٲ���I����Sn�۵����ã�____________��������ҺpH��
�ڲ���II�õ��IJ����������ձ���____________��
�۲���III����SnO�����ӷ���ʽ: ____________��
�ܲ���IV�м���SnO�Ƿ�ϴ�Ӹɾ��IJ�����____________��֤����ϴ����
�ݲ���V��������Ϊ____________ ��____________���ˡ�ϴ�ӡ����¸��
(4)ʵ����������ͼװ���Ʊ�����SnCl4(�г�װ����),��װ�ô�������ȱ��,�Ľ�������____________��

���𰸡� 50 ��Sn2+ȫ������ΪSn4+ 60% ƫ�� ��ֹSn2+������ΪSn4+�� ©��(����ͨ©��) ������ Sn2++2HCO3-= SnO��+2CO2��+ H2O ȡ���һ����Һ(ˮ��Һ) �������Թ��У����εμ��������ᡢ������������Һ���۲쵽�ް�ɫ���� ����(����) Ũ������ȴ�ᾧ ��A��Bװ�ü���������ʢ�б���ʳ��ˮ��Ũ�����ϴ��ƿ
�������������������1��Ԫ������ͬ����̼����������3���������ڵ������ڵڢ�A����
��2��Fe3+�ܰ�Sn2+����ΪSn4+�����ݹ�ϵʽ����������Ʒ�������������������ڵζ��յ�ʱ���Ӷ���������K2Cr2O7��Һ�����ƫС����3����Sn2+�ױ���������ΪSn4+���ڲ���II�ǹ��ˣ��۲���III Sn2+��HCO3-����˫ˮ�ⷴӦ���ܲ���IV�м���SnO�Ƿ�ϴ�Ӹɾ��IJ����Ǽ���ϴ��Һ���Ƿ���������������������Һ����Ũ�������½ᾧ�ɵ����������壻(4)����ͼʾװ����ȡ�����������Ȼ����ˮ���������ɵ�SnCl4������ˮ�ⷴӦ��
��������1��Ԫ������ͬ����̼����������3���������ڵ������ڵڢ�A�壬��������ԭ��������6+8+18+18=50��
��2��Fe3+�ܰ�Sn2+����ΪSn4+��������������Sn2+ȫ������ΪSn4+�����ݹ�ϵʽ3Sn ~~3 Sn2+~~3 Fe2(SO4)3~~ K2Cr2O7��n(K2Cr2O7)= 0.02L��0.1mol/L=0.002mol��n(Sn)= 0.002mol��3=0.006mol��������Ʒ��������������Ϊ![]()
���ڵζ��յ�ʱ���Ӷ���������K2Cr2O7��Һ�����ƫС��������������ƫ������3����Sn2+�ױ���������ΪSn4+���������ۿ��Է�ֹSn2+������ΪSn4+���ڲ���II�ǹ�����ʹ���������ձ���©�������������۲���III Sn2+��HCO3-����˫ˮ�ⷴӦ����Ӧ�����ӷ���ʽ��Sn2++2HCO3-=SnO��+2CO2��+H2O���ܲ���IV�м���SnO�Ƿ�ϴ�Ӹɾ��IJ����Ǽ���ϴ��Һ���Ƿ���������������������ȡ���һ����Һ(ˮ��Һ)�������Թ��У����εμ��������ᡢ������������Һ���۲쵽�ް�ɫ��������ϴ�Ӹɾ�������������Һ����Ũ�������½ᾧ�ɵ����������壬�������������IJ�������Ϊ����Ũ�������½ᾧ�����ˡ�ϴ�ӡ����¸�����(4)����ͼʾװ����ȡ�����������Ȼ����ˮ���������ɵ�SnCl4������ˮ�ⷴӦ���Ľ���������A��Bװ�ü���������ʢ�б���ʳ��ˮ��Ũ�����ϴ��ƿ��ȥ�Ȼ����ˮ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��T������2 mol X��1 mol Y�������Ϊ1L���ܱ������У���֪��2X(g)+Y(g) ![]() 2Z(s)��H=-M kJ��mol-1��10 min��ﵽƽ�⣬����0.2 mol Z�����ų�����N kJ������˵����ȷ���� ( )
2Z(s)��H=-M kJ��mol-1��10 min��ﵽƽ�⣬����0.2 mol Z�����ų�����N kJ������˵����ȷ���� ( )
A. ��10 minʱ��X�ķ�Ӧ����Ϊ0.02 mol��L-1��min-l
B. ��0��10 min�ڣ�Y�ķ�Ӧ����Ϊ![]() mol��L-1��min-l
mol��L-1��min-l
C. ��������¶���Y��ƽ��ת����
D. ��Ӧ��ƽ�����T����ͨ��ϡ����������ѹǿ����ѧ��Ӧ���ʱ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�ܱ������еķ�Ӧ:3H2(g)+N2(g)![]() 2NH3(g)��H<0��������Ӧ������ʱ��仯�������ͼ��ʾ���ж������й�t1ʱ�������仯��˵��������ȷ����
2NH3(g)��H<0��������Ӧ������ʱ��仯�������ͼ��ʾ���ж������й�t1ʱ�������仯��˵��������ȷ����

A. ����NH3Ũ�ȵ�ͬʱ��СN2Ũ�� B. ����N2��H2��Ũ��
C. ������������� D. ���ͷ�Ӧ�¶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�˽�һ����ߺϳɰ�������Ч�ʣ������о��п�����ֵ����(����)
A.���Ƹ����»��Խϴ�Ĵ���
B.Ѱ��NH3������Դ
C.���Ƶ����»��Խϴ�Ĵ���
D.�������¸�ѹ�����Ͳ��Ͻ���ϳ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС�����FeI2��Һ�л�õⵥ�ʣ������������ͼ��
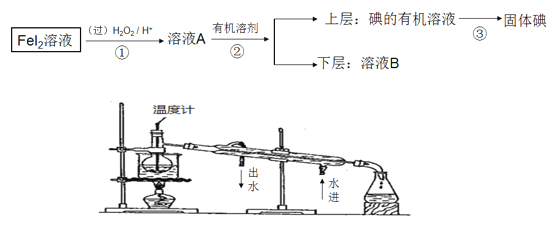
��ش�
��1���������г��ձ��⣬��Ҫ�õ��IJ���������������________________
��2����ͬѧ��ͼ����������ʵ�ֵڢ۲���������ͬѧ�������ͼ��ʵ��װ�ã���װ���е����Դ�����___________________________________
��3����ҺB�ʻ�ɫ�����˺�Fe3+�����ܻ�����I2���������һ��ʵ�鷽������֤�������ʵ�����������__________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ӻ�ˮ�п��Ի�õ�ˮ��ʳ�β�����ȡþ��������ʣ�
��1����ˮ�����ķ�����Ҫ������һ�֣���
��2���������ʲ���Ҫ������ѧ�仯���ܴӺ�ˮ�л�õ������� ������ţ���
A.Һ��
B.ʳ��
C.����
D.��ˮ
��3���Ӻ�ˮ����ȡ���þ���������£� 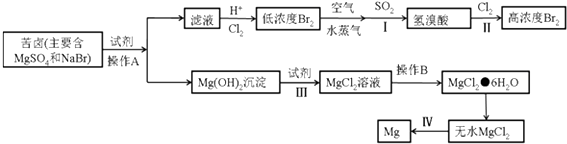
��д�����в�������ӷ���ʽ��ѧ����ʽ
����I�Ļ�ѧ����ʽ�� ��
����III�����ӷ���ʽ�� ��
����IV�Ļ�ѧ����ʽ�� ��
�ڲ���A�� �� ����B�� �� �Ӳ���II�õ�����Һ����ȡ�廹��Ҫ���еIJ����У�����ȡ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ȼ����������Ƿ�����������ɲ��õķ�����
A. �ø������ɫʯ����ֽ B. ��ʪ��ĺ�ɫ����
C. ������ͨ����������Һ�� D. �ø�����ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڷ�ӦKClO3��6HCl��KCl��3Cl2����3H2O�У��������ͱ���ԭ��ԭ�ӵ����ʵ���֮����
A. 1��6 B. 5��1 C. 6��1 D. 3��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������������������������
��4HCl(Ũ)+MnO2![]() MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O
��KC1O3+6HCl=3Cl2��+KCl+3H2O
��KMnO4+HCl(Ũ)��KCl+MnCl2+Cl2��+H2O(δ��ƽ)
��������������Ӧ���ش��й����⣺
��1����Ӧ�Ģڵ����ӷ���ʽΪ_________________________________________________________��
��2����Ӧ���У����������뻹ԭ�����������Ϊ____________________________��
��3���뽫��Ӧ����ƽ��_____KMnO4+_____HCl(Ũ)=_____KCl+____MnCl2+_____Cl2��+_____H2O
��4����Ҫ�Ƶ���ͬ��������������Ӧ�е���ת�Ƶ���Ŀ֮�ȣ����������ۣ�Ϊ___________________��
��5����֪��Ӧ��:4HCl(g)+O2![]() 2Cl2+2H2O(g)���÷�ӦҲ���Ƶ��������� MnO2��O2��KMnO4������������������ǿ������˳��Ϊ____________________________________________��
2Cl2+2H2O(g)���÷�ӦҲ���Ƶ��������� MnO2��O2��KMnO4������������������ǿ������˳��Ϊ____________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com