
����Ŀ��2019��ŵ������ѧ�������Լ��������˹�����ŵ��ɷ����˶���˹̹������͢��ķ�ͼ�Ұ�ã��Ա������Ƕ�����ӵ���з��Ĺ��ס�Ŀǰ�ȫ������ӵ����LiFePO4��أ��ṹ��ͼ��ʾ������м��Ǿۺ���ĸ�Ĥ��ֻ����Li+ͨ����
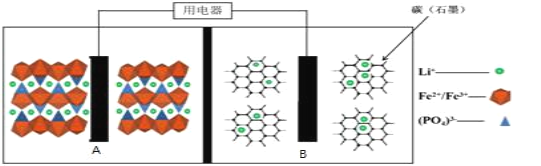
ԭ�����£�(1-x)LiFePO4+xFePO4+LixCn![]() LiFePO4+nC��
LiFePO4+nC��
����˵������ȷ���ǣ� ��
A.�ŵ�ʱ��B�缫����������Ӧ
B.�ŵ�ʱ������ת��1mol���ӣ�B�缫����������7xg
C.���ʱ��A���缫��Ӧʽ��xLiFePO4-xe-=xFePO4+xLi+
D.���ʱ��Li+��A�缫����B�缫���ƶ�
���𰸡�B
��������
���ܷ�Ӧ��(1-x)LiFePO4+xFePO4+LixCn![]() LiFePO4+nC���ŵ�ʱ��Ϊԭ��ع���ԭ����
LiFePO4+nC���ŵ�ʱ��Ϊԭ��ع���ԭ����
LixCn��C���ϼ����ߣ�ʧȥ���ӣ�����������Ӧ�����������缫��ӦʽΪLixCn-xe-=xLi++nC��FePO4��Fe���ϼ۽��ͣ��õ����ӣ�������ԭ��Ӧ�����������缫��ӦʽΪ(1-x)LiFePO4+xFePO4+xLi++xe-=LiFePO4��
���ʱ��Ϊ���ع���ԭ������ӦΪ�ŵ�ʱ������̡�
A���ŵ�ʱ��A�缫Ϊ������B�缫Ϊ����������ʧ���ӣ����ϼ����ߣ�����������Ӧ��A��ȷ��
B���ŵ�ʱ�����ݵ缫��Ӧʽ��ת��1mol����ͨ��Ĥת����1molLi+������7g��B����
C�����ʱ��AΪ����������ʧ���ӷ���������Ӧ��A���缫��Ӧʽ��xLiFePO4-xe-=xFePO4+xLi+��C��ȷ��
D�����ʱ����Ϊ���أ��������������ƶ���Li+�����ƶ���D��ȷ��
��ѡB��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���к͵ζ��������Բⶨ��������ʵ���Ũ�ȣ������ԲⶨijЩ�����ĺ�����
�ռ�����Ҫ�Ļ���ԭ�ϣ���ҵ�ռ��п��ܺ�������NaCl��Ϊ�ⶨ��ҵ�ռ���Ʒ��NaOH��������������������ʵ�飺
�ٳ�ȡ1. 000g��Ʒ�����Ƴ�250mL����Һ��
����ȡ20.00mL����Һ����0.1000mol/L��������Һ�ζ���
��1����1.000g��Ʒ���250mL����Һ�����õIJ��������У��ձ�������������ͷ�ι� _____________________________________��
��2����ȡ20.00mL����Һ���õ�������_______________________��ѡ�õ�ָʾ��Ϊ_____________________��
��3���ζ����������У��۾�Ӧע��____________________________________________���жϴﵽ�ζ��յ������Ϊ_______________________________________________��
��4����һ��ѧ���ڲⶨʱ��������ι���ͷ������ѧ���ּ���2.80mL����Һ��������ʵ�飬���ֲ���__________(���������)��
��5���ڶ���ѧ���ڵζ������У���С�Ľ���Һ������ƿ���ڱ��ϣ�һѧ������������ˮ��Һ�γ���ȥ������Ϊ��������ʹ���_______ (�ƫ�ߡ�ƫ�ͻ�û��Ӱ��)��
��6��������ѧ�����������εζ������ı����������������������е�2�εζ���ζ��ܵĶ�����ͼ��ʾ������һ����������С�
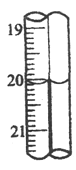
���� | �ζ�ǰ��mL�� | �ζ��� |
1 | 0.40 | 20.10 |
2 | 0.10 | ____________ |
����ѧ������õ��ռ���Ʒ��NaOH����������Ϊ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij������Ʒ�к��������Ȼ��ƣ������ⶨ��̼���Ƶ������������������ʵ�鷽����
������1����ȡһ�������Ĵ�����Ʒ����֪��ƿ��������Һ������190.720 g����������ͼװ�òⶨ������Ʒ�Ĵ��ȣ�ÿ����ͬʱ����õ�����ƽ�����������
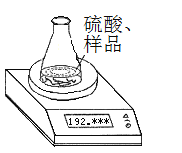
�������� | ������g�� | |
��ƿ+����+���� | ��1�� | 192.955 |
��2�� | 192.764 | |
��3�� | 192.328 | |
��4�� | 192.075 | |
��5�� | 192.075 |
��1�����㴿����Ʒ�Ĵ���ʱ�������������_____________________________����������ݣ�����������6�ζ�����ԭ����________________________________________________��
��2�����㴿����Ʒ�Ĵ���Ϊ_________________________������С������λ����
������2���ⶨ������Ʒ��1.15 g���У�Na2CO3������������һ�ַ�����������������£�
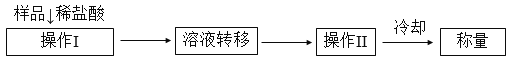
��1����Һת����__________����д�������ƣ�������II��������______________��
��2����ֱ�Ӳⶨ����������____________________��
��3���ⶨ��������Ҫ�������е�����ƽ�������ƾ��ơ�����Ҫ__________��__________���̶����г��������⣩��
��4����ת����Һʱ������Һת�Ʋ���ȫ����Na2CO3���������IJⶨ���__________������ƫС������ƫ����������������
������3��ʵ��װ����ͼ��
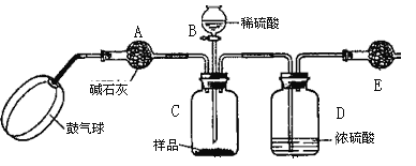
ʵ�鲽�裺
����ͼ����װ�ò���������ҩƷ��
�ڳ�������¼E������m1������ʱע����E�����ˣ���
�۰�����������Լ1���ӡ�
��������E��
�ݴ�Һ©��B�Ļ�������ϡ������ټ���C�кرջ�����
�ް�����������Լ1���ӡ�
�߳�������¼E������m2������ʱע����E�����˼�D�Ҷ˵ij��ڣ���
���ظ�����͢ߵIJ�����ֱ�����θ���ܵ������������䣬��Ϊm3��
����㡣
����պͻش����⣺
��1��C�з�����Ӧ�����ӷ���ʽΪ��______________________________________��B����������Ϊ__________���������Һ©���е����ỻ��Ũ����ͬ�����ᣬ���ԵĽ����__________������ƫ��������ƫ������������������
��2��Ũ�����������_________________����û��D����ʵ����__________������ƫ��������ƫ����������Ӱ��������
��3������ۺ͢�������____________________��____________________����������ٶ��ǿ��ٺã����ǻ������룿Ϊʲô��__________________________________________��
��4��Eװ�õĹ����Լ�Ϊ__________�����ţ�
A����ʯ�� B����ˮ�Ȼ��� C��Ũ���� D����ʯ��
��5��������Ŀ����________________________________________________��
��6�������д�����Ʒ��������������ʽΪ_____________________________________��
��7����ʵ�������������Ҫ�Ľ��ĵط�����ָ���ý�֮����˵��ԭ��
_______________________________________________________________________��
��8��ʵ�黹��������������ʵ�鷽���ⶨ�����д�������������������һ�ֲ�ͬ�Ķ���ʵ�鷽����___________________________________________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ƹ�����ñ��ʣ��������������ơ�ȡa g����������Ʒ��������ͼװ��ʵ�飬������Ʒ����(��������)��
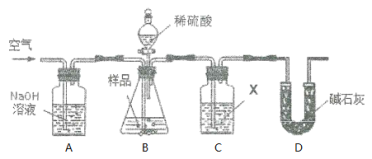
(1)��Cƿ�Լ�X��ѡ���ϣ���ͬѧ��Ϊʹ��������Ba(OH)2��Һ��������Ca(OH)2��Һ�����ʣ�������______________________��
(2)ʵ������������£���X���ü�ͬѧ�������
������A��B��ͨһ��ʱ��������ٽ���C��D��
�ڼ�ϡ���ᷴӦ��
�۷�Ӧ������ͨ�����㹻ʱ�䡣�ò�����Ŀ����________________��
�ܽ�C�����ʹ��ˡ�ϴ�ӡ�������أ���b g���塣�Դ˼��㣬����������Ʒ����Ϊ_____________��
(3)��ͬѧָ������������ʵ����ƫ��ԭ����(�û�ѧ��Ӧ����ʽ��ʾ)_____���Ľ��Ĵ�ʩ��__________________��
(4)������ͬѧ����Ľ���������ͬѧ�����Ҳ����ͨ������Cװ��ǰ����������������������ͬѧָ����˲�����ʵ������ƫС��ԭ����_______________��
(5)��ͬѧ��Ϊ���Գ���ǰ��������������ɳ���Dװ�ã���������3���������
�ٽ�Cƿ�Լ�X��Ϊ______________��
����Dװ�ú���Խ�________(��ѡ����)��
a. װ����ˮ�Ȼ��Ƶĸ���� b. װ�м�ʯ�ҵĸ����
c. װ��Ũ�����ϴ��ƿ d. װ������������Һ��ϴ��ƿ
������C֮ǰ(��C)��װ����ͨһ��ʱ�����壬�ٽ���D��֮���װ�á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ơ���������������Ҫ�Ľ�������ش�
��1�����ڿ�����ȼ�գ��õ��IJ��ﻯѧʽΪ___�����ĵ���ʽΪ___��
��2����һС������ƣ�2.3g��Ͷ��100mLˮ�У�������Ӧ�����ӷ���ʽΪ__���ɹ۲쵽��ʵ��������__(�����)��
a���Ƴ���ˮ�� b�����۳�С�� c��С���Ĵ��ζ�
��3����2��������Һ�м���5.4g�����ʳ�ַ�Ӧ��д����Ӧ�Ļ�ѧ����ʽ___��ת�Ƶ�����ĿΪ___��
��4��Fe��Cl2��һ�������·�Ӧ�����ò���Ļ�ѧʽ��___�����ò�������ˮ�����Һ����װ����֧�Թ��С���ش�
a����������һ֧�Թ��еμ�KSCN��Һ������Һ���__ɫ��
b������һ֧�Թ��еμ�NaOH��Һ������Ϊ___����Ӧ�����ӷ���ʽ��___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о�С������ú��ȵ��л���A�ϳ���֯�����̿���������M��·������(���ַ�Ӧ�Լ�������δע��)��

��֪����E�ķ���ʽΪC5H8O4���ܷ���ˮ�ⷴӦ���˴Ź���������ʾE��������2�ֲ�ͬ��ѧ��������ԭ�ӣ��������Ϊ3��1��

(R��R�䡢R�������ͬ����ͬ������)��
(1)A�����еĺ��������ŵ�������________________��
(2)D��E��Ӧ�Ļ�ѧ����ʽ��____________��
(3)A��B��Ӧ������Լ���________________��
(4)G��H��Ӧ�Ļ�ѧ����ʽ��____________��
(5)��֪1 mol E��2 mol J��Ӧ����1 mol M����M�Ľṹ��ʽ��________��
(6)E��ͬ���칹�����������ʣ�������NaHCO3��Ӧ����CO2�����ܷ���ˮ�ⷴӦ����ˮ�����֮һ�ܷ���������Ӧ�����ͬ���칹�干��________�֣���������1�ֵĽṹ��ʽ��________��
(7)J�ɺϳɸ߷��ӻ�����ø߷��ӻ�����Ľṹ��ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ����Ȼ���������ص��ƻ����ã�ˮ��Ⱦ�����̲��ݻ���BMO��Bi2MoO6����һ�ָ�Ч������������ڹ�����ⱽ�ӣ�ԭ����ͼ��ʾ������˵������ȷ���ǣ� ��

A.�ù�����O2-e-=O2-
B.�ٺ͢���BMO+��O-�����ֽ�ǿ������
C.����BMO�ܽ��ͷ�Ӧ�ķ�Ӧ�Ⱥͻ��
D.�ù���Ϊ���ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Ũ��Ϊ0.500mol/L�ı�����������Һ���ⶨδ֪Ũ�ȵ����ᡣ����ƿ�з���20.00mL�Ĵ�����Һ���ٵμ�2�η�̪��ҡ�ȡ��ñ�����������Һ�ζ���ֱ���������һ������������Һ��ָʾ������ɫ_______�����ڰ��������Һ��ɫ�������仯��ֹͣ�ζ�����¼���������������
���� | �ζ�ǰ(mL) | �ζ���(mL) |
1 | 0.40 | 21.10 |
2 | 0.10 |
��2�εζ���ζ��ܵĶ�����ͼ��ʾ�����ݱ��е����ݼ���������Ũ��Ϊ_____mol/L��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������β����ȼúβ���������������Ҫԭ��֮һ��
(1)��ҵ�����ü������ԭNO���ɼ��ٵ���������ŷš�
��֪��CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(g) ��H=-574kJ��mol1
CH4(g)+4NO(g)=2N2(g)+CO2(g)+2H2O(g) ��H =-1160kJ��mol1
����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ____________________________��
(2)��������β����Ⱦ��ԭ��Ϊ2NO(g)+2CO(g)![]() N2(g)+2CO2(g) ��H ��0������º����ܱ������г���NO��CO���ô�������õ��������±���ʾ��
N2(g)+2CO2(g) ��H ��0������º����ܱ������г���NO��CO���ô�������õ��������±���ʾ��
ʱ��/s | 0 | 1 | 2 | 3 | 4 |
c(NO)/��10-3mol��L1 | 9.00 | 4.00 | 2.00 | 1.00 | 1.00 |
c(CO)/��10-3mol��L1 | 9.00 | 4.00 | 2.00 | 1.00 | 1.00 |
��Ϊ�����β��������Ч�����ɲ�ȡ�Ĵ�ʩ��____________��д�����ּ��ɣ���
�ڴ������´ﵽƽ��ʱ������÷�Ӧ��ƽ�ⳣ��K=____________________��
(3)��ҵ��������������Һ��ͬʱ����SO2�͵�������������(NOx)���ɵõ�Na2SO3��NaHSO3��NaNO2��NaNO3����Һ������֪�������£�HNO2�ĵ��볣��ΪKa=7��10-4��H2SO3�ĵ��볣��ΪKa1=1.2��10-2��Ka2=5.8��10-8����
�ٳ����£���ͬŨ�ȵ�Na2SO3��NaNO2��Һ��pH�ϴ����________��Һ��
�ڳ����£�NaHSO3��_________�ԣ���ᡱ������С������жϵ�������________________________________________________��ͨ������˵������
(4)����(Ce)������+3��+4���ּ�̬��NO���Ա���Ce4+����Һ���գ�����NO2-��NO3-���������ʵ���֮��Ϊ1��1)���ɲ��õ�ⷨ����������Һ�е�NO2-ת��Ϊ�����ʣ�ͬʱ����Ce4+����ԭ����ͼ��ʾ��

��Ce4+�ӵ��۵�__________(����ĸ����)��������
��д�������ĵ缫��Ӧʽ______________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com