
| m(Cl2) |
| m(��������) |
| 24��2 |
| 168.5 |
| 35.5��2 |
| 168.5 |
| 24��2 |
| 168.5 |
| 35.5��2 |
| 168.5 |
| 24��2 |
| 168.5 |
| 35.5��2 |
| 168.5 |
| 24 |
| 58 |
| 24 |
| 24+71 |
| 24 |
| 24+71+32 |


 â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ʾ����һ���ݻ��̶��ĺ��������У������������һ������ܷ���壬��C��A�������������X��Y�������壬��X��Y�������ܶ���ȣ�������ֹͣ����ʱ������˵��һ����ȷ���ǣ�������
��ͼ��ʾ����һ���ݻ��̶��ĺ��������У������������һ������ܷ���壬��C��A�������������X��Y�������壬��X��Y�������ܶ���ȣ�������ֹͣ����ʱ������˵��һ����ȷ���ǣ�������| A��X��Y��Ϊ���嵥�� |
| B�����ʵ�����n��X����n��H2����n��Y�� |
| C��X����Է�����������Y����Է������� |
| D������ֹͣ����ʱ��A��B��C������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��pH=12 ��Ba��OH��2 |
| B��pH=12�İ�ˮ |
| C��0.01mol/L NaOH |
| D��0.05mol/L BaCl2 |
�鿴�𰸺ͽ���>>
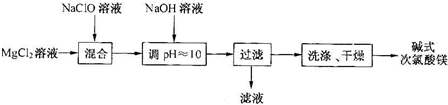
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
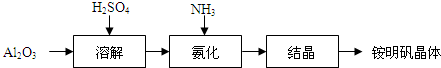
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
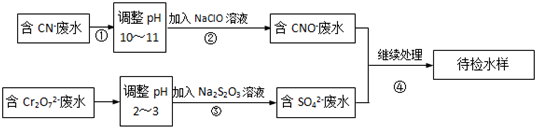
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
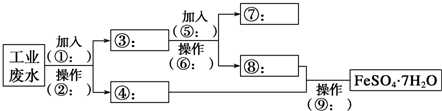
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1��2 | B��1��3 |
| C��3��1 | D��2��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com