
| 8.4g |
| 80g/mol |
| 1.44g |
| 16g/mol |
| 1.568 |
| 22.4 |
| 20.328g-15.792g |
| 18g/mol |
| 0.224L |
| 22.4L/mol |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������100mL 1.0mol/L CuSO4��Һ���ɽ�25g CuSO4?5H20����100mL����ˮ�� |
| B�����������ͷ����ˮ�У�һ��ʱ���ȡ������Һ���Թ��У���AgNO3��Һ��ϡ�����NaNO2��Һ�������ְ�ɫ������˵��������Ԫ�� |
| C����ֽ�ϲ���������ijЩ����ʱ��Ϊ�˿���ɫ�ߣ�ֻ����ɫ���ӵ����ʲſ�����ֽ������ |
| D����ѹ����ʱ������ƿ��Һ��߶Ƚ��ﵽ֧�ܿ�ʱ��Ӧ�ε�����ƿ�ϵ���Ƥ�ܣ���������ƿ֧�ܿڵ�����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��CaCO3
| ||||
| B��CaCO3+2HCl�TCaCl2+H2O+CO2�� | ||||
C��CuO+CO
| ||||
| D��CuO+H2SO4�TCuSO4+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Na+��NO3-��K+��Fe2+ |
| B��Fe2+��Na+��SO42-��K+ |
| C��K+��I-��NO3-��H+ |
| D��NH4+��NO3-��Na+��HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
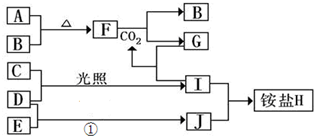
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� ���� |
��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| һ | �� | |||||||
| �� | �� | �� | �� | |||||
| �� | �� | �� | �� | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | c��һԪ�ᣩ | c��NaOH��/mol/L | �����Һ��pH |
| �� | c��HX��=0.1mol/L | 0.1 | pH=10 |
| �� | c��HY��=0.1mol/L | 0.1 | pH=7 |
| �� | c��HZ��=0.1mol/L | 0.1 | pH=9 |
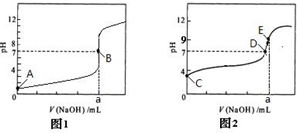
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com