
����Ŀ��(14��)ijʵ��С����0.50 mol��L- 1 NaOH��Һ��0.50mol��L- 1 ��������Һ�����к��ȵIJⶨ��
 ��������0.50mol��L- 1 ������Һ
��������0.50mol��L- 1 ������Һ
��1��������250 mL������Һ����������Ͳ��ȡ�ܶ�Ϊ1.84 g��cm- 3����������Ϊ98����Ũ���� mL��
�����ⶨϡ�����ϡ����������Һ�к��ȵ�ʵ��װ������ͼ��ʾ��
��2������A������Ϊ ��
��3��װ��������ĭ���ϵ������� ��
��4��д���÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ��(�к���Ϊ57.3 kJ��mol- 1) ��
��5��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
���±��е��¶Ȳ�ƽ��ֵΪ ����
��������Ϊ0.50mol��L- 1 NaOH��Һ��0.50mol��L- 1 ������Һ���ܶȶ���1g��cm- 3 ���кͺ�������Һ�ı�����c=" 4.18" J��(g����)- 1 �����к�����H= (ȡС�����һλ)��

������ʵ����ֵ�����57.3 kJ��mol- 1 ��ƫ�������ʵ��ƫ���ԭ������ǣ�����ĸ�� ��
A��ʵ��װ�ñ��¡�����Ч���� |
B����ȡNaOH��Һ�����ʱ���Ӷ��� |
C��һ����NaOH��Һ����ʢ�������С�ձ��� |
D�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶� |
���𰸡�������1��6.8��������2�����β������������3�����¡����ȣ�������������ʧ����4��1/2H2SO4(aq)+NaOH(aq)=1/2Na2SO4(aq)+H2O(l)��H=-57.3KJ/mol��
��5����4.0����-53. 5KJ/mol����A��D��
������������������1����ϡ��ǰ�����ʵ����ʵ������䡣(0.50mol/L��0.25L)��98g/mol="1.84" g��cm- 3��V��98�������V=6.8ml����2������A������Ϊ���β������������3��װ��������ĭ���ϵ������DZ��¡����ȣ�������������ʧ,�Dzⶨ���¶ȸ�ȷ����4�������к����ǿ�����ǿ���������ǿ���ϡ��Һ������Ӧ�����������κ�1mol��ˮʱ���ų�����������˸÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ��1/2H2SO4(aq)+NaOH(aq) =1/2Na2SO4(aq)+ H2O(l)��H=-57.3KJ/mol����5�������ᡢ�����ʵ���Ũ����ͬ�������Ƕ�Ԫ�ᣬ����ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬�����������Ӧ�ų����������ռ���м��㡣 ��ͨ���Ա��и������ݽ��й۲�ᷢ�֣���һ������ƫ��̫��Ӧ����ȥ����˱��е��¶Ȳ�ƽ��ֵΪ[��31.2��27.2������29.8��25.9������30.4��26.4��]��3="4.0��;" ��������Ϊ0.50mol��L- 1 NaOH��Һ��0.50mol��L- 1 ������Һ���ܶȶ���1g��cm- 3 ���кͺ�������Һ�ı�����c=" 4.18" J��(g����)- 1 �����к�����H=��cmt��n=(4.18��10-3��80��4.0)KJ��0.025mol="-53." 5KJ/mol. ������ʵ����ֵ�����57.3 kJ��mol- 1 ��ƫ�������ʵ��ƫ���ԭ�������A��ʵ��װ�ñ��¡�����Ч����,����ɢʧ��ʹ���ƫ�ͣ���ȷ�� B����ȡNaOH��Һ�����ʱ���Ӷ�����������ʵ���ƫ��������ƫ�࣬ʹ���ƫ�ߣ����� C��һ����NaOH��Һ����ʢ�������С�ձ��У�����ɢʧ�٣����С������ D�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ�ʹ�ڲⶨǰ�Ѿ��ɲ��ַ�����Ӧ���������ⶨֵƫ�٣���ȷ����ѡ����AD��


 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������Ͷ����Һ�У���Һ�����������(����)
A.CaCO3Ͷ�뵽HCl��Һ��
B.Na2CO3Ͷ��ϡ������
C.Fe��Ͷ��CuSO4��Һ��
D.Fe��Ͷ��ϡHCl��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪298 Kʱ���ϳɰ���ӦN2(g)��3H2(g)![]() 2NH3(g)����H����92.0 kJ��mol��1�������¶��µ�1 mol N2��3 mol H2����һ�ܱ������У��ڴ�������ʱ���з�Ӧ����÷�Ӧ�ų�������Ϊ(�ٶ�����������û��������ʧ)
2NH3(g)����H����92.0 kJ��mol��1�������¶��µ�1 mol N2��3 mol H2����һ�ܱ������У��ڴ�������ʱ���з�Ӧ����÷�Ӧ�ų�������Ϊ(�ٶ�����������û��������ʧ)
A. һ��С��92.0 kJ B. һ������92.0 kJ
C. һ������92.0 kJ D. ��ȷ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Ҫ0.1mol/LNaOH��Һ450mL��0.5mol/L��������Һ500mL��������������Һ����������ش��������⡣
��1������ͼ��ʾ�����У�����������Һ�϶�����Ҫ����_________������ţ�����ͼ�����������⣬����������Һ����Ҫ�IJ���������__________��

��2��������ƿ��ʹ�÷����У����в�������ȷ����____________
A��ʹ������ƿǰ�����Ƿ�©ˮ |
B������ƿ��ˮϴ�������ô�����Һϴ�� |
C��������Һʱ����������ǹ��壬�ѳƺõĹ�����ֽ��С�ĵ�������ƿ�У�������ˮ���ӽ��̶���1~2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߡ� |
D��������Һʱ����������Һ�壬����Ͳȡ�����ò�����������������ƿ�У�������ˮ���̶���1~2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߡ� |
E���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȡ�
��3�����ݼ�����������ƽ��ȡ������Ϊ______g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��_____0.1mol/L��������������С������������������
��4�����ݼ����֪��������Ͳ��ȡ��������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������Ϊ_______mL�����ʵ������15mL��20mL��50mL��Ͳ��Ӧѡ��______mL��Ͳ��á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��WΪԭ��������������Ķ���������Ԫ�ء�X�ֱ���Y��Z��W����γ���������ͬ�ļס��ҡ������ַ��ӡ���Ϊ��ɫ���壬�����������ɫ������ˮ��Һ�ɿ�ʴ������
������������ͼת����ϵ��

����˵���������
A. ����Ԫ���γɵĵ�����W����������ǿ
B. �ס��ҡ����зе���ߵ��DZ�
C. �׳����������
D. �ס��ҷ��Ӿ�ֻ�����Թ��ۼ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ�����ý�����ȷ����

A. ������Һ��ɫ��ȥ��ԭ���ǣ�CH3COOC2H5 + NaOH =CH3COONa + C2H5OH
B. ������Һ����ԭ���ǣ�CH3COO�� + H2O ![]() CH3COOH + H+
CH3COOH + H+
C. ��ʵ��١��ڡ����Ʋ⣬���к�ɫ��ȥ��ԭ��������������ȡ�˷�̪
D. ���к�ɫ��ȥ֤���Ҳ�С�Թ����ռ��������������л�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����úֱ����Ϊȼ��ȼ�գ�������Ч�ʽϵͣ��Ҳ����̳���������������������ʣ�������صĻ�����Ⱦ��ú�ĸ��������ú�������ʡ�������Ҫ����ԭ�ϡ�������Ⱦ���ŷ�������Ч��ʩ֮һ��ij��ѧѧϰС����ʵ����������̽��ú����������װ����ͼ��ʾ����ش��й����⣺

(1)ú�����������_______��
(2)ʢ����ˮ���ձ���������__________________
(3)ʵ�������дְ�ˮ���ɵIJ�����___
(4)��֪CO����ʹ��ˮ��ɫ�������Ӿ�֧�Թ�֧�ܿڴ��ݳ�������ͨ����ˮ�У�������ˮ��ɫ����˵��ú�ĸ��������_______________________
(5)��ȼβ�����������ɫΪ________________________
(6)��ú�����з���������ױ������ױ���ʵ�鷽����_________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼʾ�У�AΪһ�ֳ����ĵ��ʣ�B��C��D��E�Ǻ�AԪ�صij�����������ǵ���ɫ��Ӧ��Ϊ��ɫ��
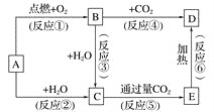
����д���пհף�
(1)д����ѧʽ��A____��B____��C_____��D____��E____��
(2)���Ϸ�Ӧ�У�����������ԭ��Ӧ����____________(��д���)��
(3)A��C��Ӧ�����ӷ���ʽ��________��
(4) B��C��Ӧ�Ļ�ѧ����ʽ��____________��
(5)E��D��Ӧ�Ļ�ѧ����ʽ��_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ũ�Ⱦ�Ϊ0.1 mol��L��1��������Һ�������ᡢ�ڴ��ᡢ���������ơ����Ȼ�李��ݴ���李���������李��߰�ˮ����ش��������⣺
��1���١��ڡ��ۡ���������Һ����ˮ�������H��Ũ���ɴ�С��˳����(�����)___________��
��2���ܡ��ݡ��ޡ���������Һ��NH![]() Ũ���ɴ�С��˳����(�����)_______________��
Ũ���ɴ�С��˳����(�����)_______________��
��3�����ۺܰ͢������1��2��Ϻ��Һ�и�����Ũ���ɴ�С��˳���ǣ�__________________��
��4����֪t ��ʱ��KW��1��10��13����t ��(�����������������)________25�档��t ��ʱ��pH��11��NaOH��Һa L��pH��1��H2SO4��Һb L���(���Ի�Ϻ���Һ����ı仯)�������û����Һ��pH��2����a��b��________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com