
��Ȫ��һ�ֳ�����ʵ������(����ͼ)�������ԭ���Ǵ���ѹǿ�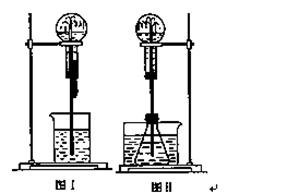
��1��ͼ��Ϊ��ѧ��ѧ�����õ���Ȫʵ��װ�á�����ƿ�г����������壬��ͷ�ιܼ��ձ��зֱ�ʢ��Һ�塣��������в������γ���Ȫ����(������ĸ) ��
A.HCl ��H2O B.O2��H2O
C.SO2��NaOH��Һ D.CO2��NaOH��Һ
��2��ijѧ������˼��������Ȫ�������취���������ͼ����ʾ�� װ�á�
��ͼ�����ƿ�У��ֱ�����������������ʣ���Ӧ����ܲ��� ��Ȫ����(������ĸ) ��
A��CaCO3��ϡH2SO4 B��NaOH��ϡHCl
C��Zn��ϡHCl D��NaCl��ϡHNO3
��3���Ƚ�ͼ���ͼ������װ�ã��Ӳ�����Ȫ��ԭ�����������ߵIJ�ͬ������ ��
��4�������г�����������Ȫ����ɽ������ԭ�����������ͼ��ͼ��װ�õ�ԭ�����ơ�
��1��B��2�� C��3�����ߵIJ�ͬ������ͼһԲ����ƿ�ڲ�ѹǿ��С��С�ڴ���ѹ��ͼ����ƿ��ѹǿ����4��ͼ��
���������������1��A.�Ȼ���������ˮ����ƿ�е�����ѹǿ�ͻ��С�������γ���Ȫ��B.������������ˮ����ƿ�е�����ѹǿ���������Եı仯���ʲ����γ���Ȫ��C.SO2������ˮ����ƿ�е�����ѹǿ�ͻ��С�������γ���Ȫ��D.������̼�ܺ�����������Һ��Ӧ����ƿ�е�����ѹǿ�ͻ��С�������γ���Ȫ��
��2��A.CaCO3��ϡH2SO4��Ӧ���ɶ�����̼���壬������̼����ˮ�������γ���Ȫʵ�飻B.NaOH��ϡHCl �䷴Ӧ�����������壬�����ڵ�ѹǿû�б仯�������γ���Ȫ��C.Zn��ϡ���ᷴӦ������������ʹ��ƿ��ѹǿ������ƿ��ѹǿ���γ���Ȫ��D.NaCl��ϡHNO3����Ӧ����ƿ����ƿ��ѹǿ��ȣ������γ���Ȫ��
��3�����ߵIJ�ͬ������ͼһԲ����ƿ�ڲ�ѹǿ��С��С�ڴ���ѹ��ͼ����ƿ��ѹǿ����
��4�������г�����������Ȫ����ɽ������ԭ�������ڲ�ѹǿ�����γɵġ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Դ��һ����Ҫ�������Դ���������ֿɲ���H2�Ļ�������ҡ���6.00 g��������ȫ�ֽ⣬ֻ�õ�һ�ֶ�����Ԫ�صĽ������ʺ�6.72 LH2(������ɱ�״��)������ˮ��ӦҲ�ܲ���H2��ͬʱ������һ�ְ�ɫ������ð�ɫ����������NaOH��Һ�����������ڴ��������¿ɷֽ�õ�H2����һ�ֵ�������������ڱ�״̬�µ��ܶ�Ϊ1.25 g/L����ش��������⣺
(1)�Ļ�ѧʽ��_________���ҵĵ���ʽ��__________��
(2)����ˮ��Ӧ�Ļ�ѧ����ʽ��__________________________________��
(3)����������þ��Ӧ�IJ�����_______(�û�ѧʽ��ʾ)��
(4)���ڼ�����������CuO��Ӧ������Cu���������д���÷�Ӧ�Ļ�ѧ����ʽ_________�������������Cu�п��ܻ�����Cu2O�������ʵ�鷽����֤֮______________��(��֪Cu2O+2H+==Cu+Cu2++H2O)
(5)������֮��_______(����ܡ������ܡ�)������Ӧ����H2���ж�������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ�����ס�������װ�в�ͬ���ʵ���Ͳ�õ�������������������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ�ͬʵ��(������ͬ��ͬѹ�²ⶨ)���Իش��������⣺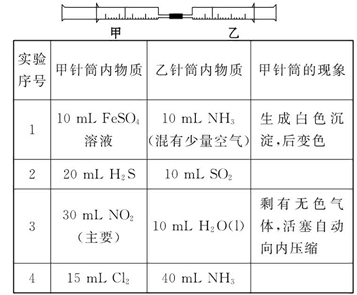
(1)ʵ��1�У��������ձ�Ϊ_______ɫ��д��������ɫ�Ļ�ѧ����ʽ_____________________��
(2)ʵ��2����Ͳ�ڵ������ǣ���_______���ɣ���Ͳ����_______�ƶ�(����⡱�������ڡ�����)����Ӧ�����Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��_______��Һ�С�
(3)ʵ��3�У����е�30 mL������NO2��N2O4�Ļ�����壬��ô�������ʣ�����ɫ������_______��д��NO2��H2O��Ӧ�Ļ�ѧ����ʽ____________________________��
(4)ʵ��4�У���֪��3Cl2+2NH3=N2+6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�仯Ϊ_______�������Ͳ��ʣ����������ԼΪ_______mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣�ijЩ��ѧ��Ӧ���ñ�ʾ��δ��ƽ����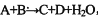 ����ش��������⣺
����ش��������⣺
��1����A��C��D��������Ԫ�أ���A����Ԫ�صĻ��ϼ۽���C��D֮�䣬д���÷�Ӧ�����ӷ���ʽ��_________________________________________________��
��2����AΪ�Ϻ�ɫ������DΪ��ɫ�̼������壬��д��������ʽ�Ļ�ѧ����ʽ��______________________________________________________________��
��3����C��D��Ϊ���壨����C����ɫ���ҷ��Ӿ�����ͬ��ԭ�Ӹ����ȣ��������ʽ��ѧ����ʽ�ǣ�_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼ��ʾװ�ý���ʵ�飨�г�װ������ȥ������ش��������⣺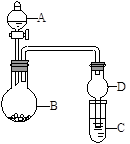
��1����A��ΪŨ���ᣬB��Ϊͭ���ʣ�C��Ϊ����������Һ���ֽ�Ũ�������B�У���B�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����A��ΪŨ��ˮ��B��Ϊ��ʯ�ң�C��Ϊ������Һ��
�ٽ�Ũ��ˮ��ε���B�У��ɲ�������������ԭ������� _ ������ţ���
a����ʯ�Һ�Ũ��ˮ��Ӧ��������������������ˮ
b����ʯ�Һ�ˮ��Ӧ������ˮ��ʹ�������ܽ�������
c����Ӧ�ų������ȣ�ʹ������ˮ���ܽ�����Խ���
�� C��ͨ���������ʱ����Ӧ�����ӷ���ʽΪ ��
��3����A��Ϊˮ��B��Ϊ�������ƣ�C��Ϊ���Ե��۵⻯����Һ����ˮ����B�к�B�е�ʵ������Ϊ ����C����Һ��Ϊ��ɫ����C�з�����Ӧ�����ӷ���ʽΪ_ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
X��Y��Z��Q��R�����ֶ�����Ԫ�أ�ԭ��������������X��Y��Ԫ����������������֮�;�Ϊ0��Q��Xͬ���壻Z��R�ֱ��ǵؿ��к�����ߵķǽ���Ԫ�غͽ���Ԫ�أ���ش��������⣺
��1������Ԫ��ԭ�Ӱ뾶�ɴ�С��˳����(дԪ�ط���)
��2��X��Y��Z���γɶ��ֻ�������мȺ����Լ��ֺ��Ǽ��Լ�������Է���������С��������(д����)
��������ʹ���Ը��������Һ��ɫͬʱ����һ�������г����л���˹��̵����ӷ���ʽΪ
��3��������ijЩԪ����ɵĻ�����A��B��C��D������ת����ϵ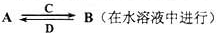
����C������ˮ�����Ե����壻D�ǵ���ɫ���壮
д��C�Ľṹʽ ��
�����A��B��������Ԫ����ɣ�BΪ���Բ��������Aת��ΪB�����ӷ���ʽ
�����A������Ԫ����ɣ�B������Ԫ����ɣ�A��B��Һ���Լ��ԣ������ӷ���ʽ��ʾA��Һ�Լ��Ե�ԭ�� �� A��BŨ�Ⱦ�Ϊ0��1mol/L�Ļ����Һ�У�����Ũ���ɴ�С��˳���� �������£��ڸ���Һ�еμ�ϡ����������ʱ�����ʵ���Ҫ�ɷ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ԫ��A��B��C��D��E��Ԫ�����ڱ��е�λ������ͼ��ʾ��B���γɻ�������������Ԫ�ء�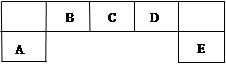
��ش��������⣺
��1������D��Ԫ�����ڱ��е�λ�ã�_____________________
��2���Ƚ�A��C��DԪ�ؼ����Ӱ뾶�Ĵ�С��______��______��______(��������)
��3��E���⻯����������������ˮ����ļ��ι����ܷ�����Ӧ������һ�����嵥�ʣ���Ӧ�Ļ�ѧ��Ӧ����ʽΪ_______________________________________________.
��4��F��Dͬ���������ڣ���˵��D���⻯���F���⻯���ȶ��ĸ���ԭ��______________��
�ø�����������Һ̬ˮʱ��һ��ˮ�������ͷų�һ�����ӣ�ͬʱ����һ�־��н�ǿ�������Ե������ӣ���д�������ӵĵ���ʽ��________��д������������F�⻯���ˮ��Һ��Ӧ�����ӷ���ʽ��__________________________________________________________��
��5����Fe��Cu �Ļ�����м���һ������C������������ˮ����ϡ��Һ����ַ�Ӧ��ʣ�����m1g,�������м���һ������ϡ���ᣬ��ַ�Ӧ��ʣ�����m2g������˵����ȷ���ǣ� ��
A������ϡ����ǰ������ϡ��������Һ�п϶�����Cu2+
B������ϡ����ǰ������ϡ��������Һ�п϶�����Fe2+
C��m1һ������m2
D��ʣ�����m1g��һ���е���ͭ��ʣ�����m2g��һ��û�е���ͭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijЩ��ѧ��Ӧ������ʽ��ʾ��δ��ƽ����A+B��C+D+H2O
��ش��������⣺
��1����A��C��D��������Ԫ�أ���A����Ԫ�صĻ��ϼ۽���C��D֮�䣬д���÷�Ӧ�����ӷ���ʽ��___________________________________________��
��2����CΪ�Ȼ��ƣ�D����ʹ����ʯ��ˮ����ǵ���ζ���壬��A��B������ǣ�����������A________________��________________ , B______________________��
��3����AΪ�Ϻ�ɫ������DΪ��ɫ�̼������壬��д��������ʽ�Ļ�ѧ����ʽ��____________________________________________��
��4����C��D��Ϊ�����ҷ��Ӿ�����ͬ��ԭ�Ӹ����ȣ��������ʽ��ѧ����ʽ�ǣ�____________________________________________��
��5����AΪ�������ƣ�BΪ���ᣬ��C��____________��D��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ijԪ��ԭ�ӵ��������1�����ӣ����Ԫ�ز�������
| A��IA��Ԫ�� | B������Ԫ�� | C���ǽ���Ԫ�� | D������Ԫ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com