
ЎҫМвДҝЎҝI.СМөАЖшЦРөДNO2КЗЦчТӘөДҙуЖшОЫИҫОпЦ®Т»Ј¬ОӘБЛјаІвЖдә¬БҝЈ¬СЎУГИзПВјмІв·Ҫ·ЁЎЈ»ШҙрПВБРОКМвЈә
Ҫ«VLЖшСщНЁИлККБҝЛб»ҜөДH2O2ИЬТәЦРЈ¬К№NO2НкИ«ұ»Сх»ҜіЙNO3-Ј¬јУЛ®ПЎКНЦБ100.00mLЎЈБҝИЎ20.00mLёГИЬТәЈ¬јУИлv1mLc1molЎӨLЈӯ1 FeSO4ұкЧјИЬТә(№эБҝ)Ј¬ід·Ц·ҙУҰәуЈ¬УГc2molЎӨLЈӯ1 K2Cr2O7ұкЧјИЬТәөО¶ЁКЈУаөДFe2Ј«Ј¬ЦХөгКұПыәДv2mLЎЈ
(1)NO2ұ»H2O2Сх»ҜОӘNO3-өДАлЧУ·ҪіМКҪОӘ____________________________________ЎЈ
(2)јУЛ®ПЎКНөҪ100.00mlЛщУГөДІЈБ§ТЗЖчіэБҝНІЎўЙХұӯЎўІЈБ§°фЎўҪәН·өО№ЬНвЈ¬»№РиТӘ__________________ЎЈ
(3)өО¶Ё№эіМЦР·ўЙъПВБР·ҙУҰЈә
3Fe2Ј«Ј«NO3-Ј«4HЈ«=NOЎьЈ«3Fe3Ј«Ј«2H2O
Cr2O72-Ј«6Fe2Ј«Ј«14HЈ«=2Cr3Ј«Ј«6Fe3Ј«Ј«7H2O
ФтЖшСщЦРNO2өДә¬БҝОӘ___________mg/LЎЈ
(4)ПВБРІЩЧч»бК№өО¶ЁҪб№ыЖ«ёЯөДКЗ____
AЈ®өО¶Ё№ЬОҙУГұкЧјТәИуПҙ BЈ®Ч¶РОЖҝПҙҫ»әу»№ҙжБфЙЩБҝөДЛ®
CЈ®өО¶Ё№ЬөО¶ЁЗ°¶БКэХэИ·Ј¬өО¶Ёәуё©КУ¶БКэ DЈ®FeSO4ұкЧјИЬТәІҝ·ЦұдЦК
IIЈ®іЈОВПВЈ¬УГ·УМӘЧчЦёКҫјБЈ¬УГ0.10molЎӨLЈӯ1 NaOHИЬТә·ЦұрөО¶Ё20.00mLЕЁ¶ИҫщОӘ0.10molЎӨLЈӯ1өД CH3COOHИЬТәәНHCNИЬТәЛщөГөО¶ЁЗъПЯИзНјЎЈ
ЈЁТСЦӘЈәCH3COOHЎў HCNөДөзАлЖҪәвіЈКэ·ЦұрОӘ1.75ЎБ10-5Ўў6.4ЎБ10-10Ј©
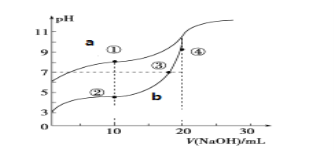
(1)Нј___ЈЁa»тbЈ©КЗNaOHИЬТәөО¶ЁHCNИЬТәөДpHұд»ҜөДЗъПЯЈ¬ЕР¶ПөДАнУЙКЗ_______________________ЎЈ
(2)өгўЫЛщКҫИЬТәЦРЛщә¬АлЧУЕЁ¶ИөДҙУҙуөҪРЎөДЛіРтЈә_______________________ЎЈ
(3)өгўЩәНөгўЪЛщКҫИЬТәЦРЈәc(CH3COOЈӯ)Јӯc(CNЈӯ)___c(HCN)Јӯc(CH3COOH)ЈЁМоЎ°>Ўў<»т=ЎұЈ©
(4)өгўЪўЫўЬЛщКҫөДИЬТәЦРЛ®өДөзАліМ¶ИУЙҙуөҪРЎөДЛіРтКЗЈә____________________ЎЈ
Ўҫҙр°ёЎҝ2NO2Ј«H2O2= 2NO3ЈӯЈ«2HЈ« 100mLИЭБҝЖҝ ![]() CD a HCNөДөзАлЖҪәвіЈКэРЎЈ¬Н¬ЕЁ¶ИЈ¬ЖдөзАліцөДЗвАлЧУЕЁ¶ИРЎЈ¬pHЦөҙу c(CH3COOЈӯ)= c(Na+) > c(OHЈӯ) = c(H+) = ўЬўЫўЪ
CD a HCNөДөзАлЖҪәвіЈКэРЎЈ¬Н¬ЕЁ¶ИЈ¬ЖдөзАліцөДЗвАлЧУЕЁ¶ИРЎЈ¬pHЦөҙу c(CH3COOЈӯ)= c(Na+) > c(OHЈӯ) = c(H+) = ўЬўЫўЪ
ЎҫҪвОцЎҝ
I(1)NO2ұ»H2O2Сх»ҜОӘNO3ЈӯөДАлЧУ·ҪіМКҪОӘ2NO2Ј«H2O2= 2NO3ЈӯЈ«2HЈ«ЎЈ
(2)јУЛ®ПЎКНөҪ100.00mlЛщУГөДІЈБ§ТЗЖчіэБҝНІЎўЙХұӯЎўІЈБ§°фЎўҪәН·өО№ЬНвЈ¬»№РиТӘ100mLИЭБҝЖҝЎЈ
(3)ПИјЖЛгЦШёхЛбёщАлЧУПыәДөГСЗМъАлЧУөДОпЦКөДБҝЈ¬ФЩјЖЛгПхЛбёщПыәДөГСЗМъАлЧУөДОпЦКөДБҝЈ¬ФЩјЖПхЛбёщөДОпЦКөДБҝјҙ¶юСх»ҜөӘөДОпЦКөДБҝЈ¬ФЩјЖЛгvLЖшМеЦР¶юСх»ҜөӘөДә¬БҝЎЈ
(4)AСЎПоЈ¬өО¶Ё№ЬОҙУГұкЧјТәИуПҙЈ¬ПыәДөГұкТәМе»эФцҙуЈ¬ЦШёхЛбёщМе»э¶БКэЖ«ҙуЈ¬ЦШёхЛбёщПыәДСЗМъАлЧУЖ«ҙуЈ¬ПхЛбёщПыәДСЗМъАлЧУЖ«РЎЈ¬өО¶ЁҪб№ыЖ«өНЈ»BСЎПоЈ¬Ч¶РОЖҝПҙҫ»әу»№ҙжБфЙЩБҝөДЛ®Ј¬Ів¶ЁҪб№ыІ»ұдЈ»CСЎПоЈ¬өО¶Ё№ЬөО¶ЁЗ°¶БКэХэИ·Ј¬өО¶Ёәуё©КУ¶БКэЖ«РЎЈ¬ЦШёхЛбёщПыәДСЗМъАлЧУЖ«РЎЈ¬ПхЛбёщПыәДСЗМъАлЧУЖ«ҙуЈ¬өО¶ЁҪб№ыЖ«ёЯЈ»DСЎПоЈ¬FeSO4ұкЧјИЬТәІҝ·ЦұдЦКЈ¬Ів¶ЁЦШёхЛбёщМе»эЖ«РЎЈ¬ЦШёхЛбёщПыәДСЗМъАлЧУЖ«РЎЈ¬ПхЛбёщПыәДСЗМъАлЧУЖ«ҙуЈ¬Ів¶ЁҪб№ыЖ«ёЯЎЈ
IIЈ®(1)НјёщҫЭөзАлЖҪәвіЈКэҝЙЦӘЈ¬Н¬ЕЁ¶ИHCNөДpHөзАліМ¶ИРЎЈ¬pHҙуЈ¬ТтҙЛЗъПЯaКЗNaOHИЬТәөО¶ЁHCNИЬТәөДpHұд»ҜөДЗъПЯЎЈ
(2)өгўЫКЗҙЧЛбУлЗвСх»ҜДЖ·ҙУҰіКЦРРФЈ¬ёщҫЭөзәЙКШәгәНИЬТәіКЦРРФөГіцАлЧУЕЁ¶ИөДҙУҙуөҪРЎөДЛіРтЎЈ
(3)өгўЩИЬЦКОӘNaCNЎўHCNЗТЕЁ¶ИПаөИЈ¬өгўЪИЬЦКОӘCH3COOHЎўCH3COONaЗТЕЁ¶ИПаөИЈ¬өгўЩЎўөгўЪөДИЬЦКөДЕЁ¶И¶јПаН¬Ј¬ёщҫЭОпБПКШәгөГұдРОөГіцҪбВЫЎЈ
(4)ҙУөгўЪөҪөгўЬ№эіМЦРЈ¬ИЬТәЦРТЦЦЖФЩ·ўЙъЛбјоЦРәН·ҙУҰЈ¬Лб¶ФЛ®өДөзАліМ¶ИЦрҪҘјхРЎЈ¬№КЛ®өДөзАліМ¶ИЦрҪҘФцҙуЎЈ
I(1)NO2ұ»H2O2Сх»ҜОӘNO3ЈӯөДАлЧУ·ҪіМКҪОӘ2NO2Ј«H2O2= 2NO3ЈӯЈ«2HЈ«Ј¬№Кҙр°ёОӘЈә2NO2Ј«H2O2= 2NO3ЈӯЈ«2HЈ«ЎЈ
(2) јУЛ®ПЎКНөҪ100.00mlЛщУГөДІЈБ§ТЗЖчіэБҝНІЎўЙХұӯЎўІЈБ§°фЎўҪәН·өО№ЬНвЈ¬»№РиТӘ100mLИЭБҝЖҝЈ¬№Кҙр°ёОӘЈә100mLИЭБҝЖҝЎЈ
(3)Cr2O72ЈӯЈ« 6Fe2Ј«Ј«14HЈ«=2Cr3Ј«Ј«6Fe3Ј«Ј«7H2O
1mol 6mol
c2molЎӨLЈӯ1ЎБv2ЎБ10-3 L xmol
![]()
x = 6c2v2ЎБ10-3 mol
БтЛбСЗМъөДОпЦКөДБҝn(FeSO4) = c1molЎӨLЈӯ1ЎБv1ЎБ10-3 L= c1v1ЎБ10-3 mol
ПхЛбёщСх»ҜБтЛбСЗМъөДОпЦКөДБҝОӘn(FeSO4) = c1v1ЎБ10-3 mol - 6c2v2ЎБ10-3 mol =(c1v1- 6c2v2) ЎБ10-3 mol
3Fe2Ј«Ј« NO3ЈӯЈ«4HЈ«=NOЎьЈ«3Fe3Ј«Ј«2H2O
3mol 1mol
(c1v1- 6c2v2) ЎБ10-3 mol ymol
![]()
![]()
ТтҙЛVLёГЖшМеNO2өДОпЦКөДБҝ![]() Ј¬ЖшСщЦРNO2өДЦКБҝОӘ
Ј¬ЖшСщЦРNO2өДЦКБҝОӘ![]() =
=![]() Ј¬ФтЖшСщЦРNO2өДә¬Бҝ
Ј¬ФтЖшСщЦРNO2өДә¬Бҝ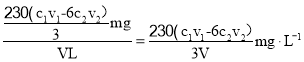 Ј¬№Кҙр°ёОӘЈә
Ј¬№Кҙр°ёОӘЈә![]() ЎЈ
ЎЈ
(4)AСЎПоЈ¬өО¶Ё№ЬОҙУГұкЧјТәИуПҙЈ¬ПыәДөГұкТәМе»эФцҙуЈ¬ЦШёхЛбёщМе»э¶БКэЖ«ҙуЈ¬ЦШёхЛбёщПыәДСЗМъАлЧУЖ«ҙуЈ¬ПхЛбёщПыәДСЗМъАлЧУЖ«РЎЈ¬өО¶ЁҪб№ыЖ«өНЈ»
BСЎПоЈ¬Ч¶РОЖҝПҙҫ»әу»№ҙжБфЙЩБҝөДЛ®Ј¬Ів¶ЁҪб№ыІ»ұдЈ»
CСЎПоЈ¬өО¶Ё№ЬөО¶ЁЗ°¶БКэХэИ·Ј¬өО¶Ёәуё©КУ¶БКэЖ«РЎЈ¬ЦШёхЛбёщПыәДСЗМъАлЧУЖ«РЎЈ¬ПхЛбёщПыәДСЗМъАлЧУЖ«ҙуЈ¬өО¶ЁҪб№ыЖ«ёЯЈ»
DСЎПоЈ¬FeSO4ұкЧјИЬТәІҝ·ЦұдЦКЈ¬Ів¶ЁЦШёхЛбёщМе»эЖ«РЎЈ¬ЦШёхЛбёщПыәДСЗМъАлЧУЖ«РЎЈ¬ПхЛбёщПыәДСЗМъАлЧУЖ«ҙуЈ¬Ів¶ЁҪб№ыЖ«ёЯЈ»
№Кҙр°ёОӘCDЎЈ
IIЈ®(1)НјёщҫЭөзАлЖҪәвіЈКэҝЙЦӘЈ¬Н¬ЕЁ¶ИПВЈ¬HCNөДөзАліМ¶ИРЎЈ¬pHҙуЈ¬ТтҙЛЗъПЯaКЗNaOHИЬТәөО¶ЁHCNИЬТәөДpHұд»ҜөДЗъПЯЈ¬№Кҙр°ёОӘЈәaЈ»HCNөДөзАлЖҪәвіЈКэРЎЈ¬Н¬ЕЁ¶ИЈ¬ЖдөзАліцөДЗвАлЧУЕЁ¶ИРЎЈ¬pHЦөҙуЎЈ
(2)өгўЫКЗҙЧЛбУлЗвСх»ҜДЖ·ҙУҰіКЦРРФЈ¬ёщҫЭөзәЙКШәгәНИЬТәіКЦРРФөГіцАлЧУЕЁ¶ИөДҙУҙуөҪРЎөДЛіРтЈәc(CH3COOЈӯ)= c(Na+) > c(OHЈӯ) = c(H+)Ј¬№Кҙр°ёОӘЈәc(CH3COOЈӯ)= c(Na+) > c(OHЈӯ) = c(H+)ЎЈ
(3)өгўЩИЬЦКОӘNaCNЎўHCNЗТЕЁ¶ИПаөИЈ¬өгўЪИЬЦКОӘCH3COOHЎўCH3COONaЗТЕЁ¶ИПаөИЈ¬өгўЩЎўөгўЪөДИЬЦКөДЕЁ¶И¶јПаН¬Ј¬ёщҫЭОпБПКШәгөГұдРОөГіцЈәc(CH3COOЈӯ)Јӯc(CNЈӯ) = c(HCN)Јӯc(CH3COOH)Ј¬№Кҙр°ёОӘЈә=ЎЈ
(4)ҙУөгўЪөҪөгўЬ№эіМЦРЈ¬ИЬТәЦРТЦЦЖФЩ·ўЙъЛбјоЦРәН·ҙУҰЈ¬Лб¶ФЛ®өДөзАліМ¶ИЦрҪҘјхРЎЈ¬№КЛ®өДөзАліМ¶ИЦрҪҘФцҙуЈ¬ТтҙЛИЬТәЦРЛ®өДөзАліМ¶ИУЙҙуөҪРЎөДЛіРтКЗЈәўЬўЫўЪЈ¬№Кҙр°ёОӘЈәўЬўЫўЪЎЈ


 ГыРЈБӘГЛіеҙМҫнПөБРҙр°ё
ГыРЈБӘГЛіеҙМҫнПөБРҙр°ё ГыРЈМб·ЦТ»ҫнНЁПөБРҙр°ё
ГыРЈМб·ЦТ»ҫнНЁПөБРҙр°ё ҝОіМҙпұкІвКФҫнҙі№Ш100·ЦПөБРҙр°ё
ҝОіМҙпұкІвКФҫнҙі№Ш100·ЦПөБРҙр°ё РВҫнНхЖЪД©іеҙМ100·ЦПөБРҙр°ё
РВҫнНхЖЪД©іеҙМ100·ЦПөБРҙр°ё
| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝідВъNO2әНO2»мәПЖшМе30mLөДКФ№Ьө№БўУЪЛ®ЦРЈ¬ЧоЦХКФ№ЬЦРКЈУа5mLЖшМеЈ¬ФтФӯКФ№ЬЦРNO2әНO2өДМе»эұИҝЙДЬКЗ
A.1©U1B.3©U1C.5©U1D.9©U1
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПВБР·ҙУҰОп°ҙЛщёшОпЦКөДБҝЕдұИҪшРР·ҙУҰЈ¬ЖдЦР№ММеНкИ«·ҙУҰөДКЗЈЁ Ј©
A.nЈЁCuЈ©ЈәnЈЁHNO3ЕЁЈ©=1Јә4B.nЈЁFeЈ©ЈәnЈЁHNO3ЕЁЈ©=1Јә2
C.nЈЁCЈ©ЈәnЈЁH2SO4ЕЁЈ©=1Јә2D.nЈЁMnO2Ј©ЈәnЈЁHClЕЁЈ©=1Јә4
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝҪрКфІДБПФЪИХіЈЙъ»оЎўЙъІъЦРУРЧЕ№г·әөДФЛУГЈ¬ПВБР№ШУЪҪрКфөДЛө·ЁІ»ХэИ·өДКЗ
A.№ӨТөЙПҪрКфMgЎўAl¶јКЗУГөзҪвИЫИЪөДВИ»ҜОпЦЖөГөД
B.әПҪрөДРФЦКУлЖдіЙ·ЦҪрКфөДРФЦКІ»НкИ«ПаН¬
C.ҪрКфТұБ¶өДұҫЦККЗҪрКфСфАлЧУөГөҪөзЧУұдіЙҪрКфФӯЧУ
D.ФҪ»оЖГөДҪрКфФҪДСТұБ¶
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝ°ұҙЯ»ҜСх»Ҝ·ЁЦЖПхЛбКЗ№ӨТөЙъІъПхЛбөДЦчТӘНҫҫ¶Ј¬ДіН¬С§АыУГёГФӯАнФЪКөСйКТМҪҫҝПхЛбөДЦЖұёәНРФЦКЈ¬ЙијЖБЛИзНјЛщКҫЧ°ЦГЎЈ
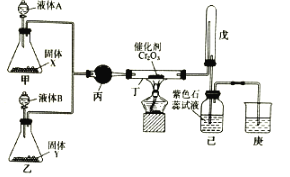
»ШҙрПВБРОКМвЈә
ЈЁ1Ј©јЧЎўТТБҪЧ°ЦГ·ЦұрЦЖИЎ°ұЖшәНСхЖшЎЈКўЧ°ТәМеAЎўBөДТЗЖчГыіЖОӘ__ЎЈИф№ММеXКЗNaOHЈ¬ФтТәМеAОӘ__ЈЁМоГыіЖЈ©Ј»ИфYКЗөӯ»ЖЙ«№ММеЎўBОӘҙҝҫ»ОпЈ¬ФтТәМеBОӘ__ЈЁМо»ҜС§КҪЈ©Ј»Иф№ММеYКЗMnO2Ј¬ФтТәМеBОӘ__ЈЁМо»ҜС§КҪЈ©ЎЈ
ЈЁ2Ј©¶ЎЦР·ўЙъ·ҙУҰөД»ҜС§·ҪіМКҪОӘ__ЎЈ
ЈЁ3Ј©Ч°ЦГОмөДЧчУГКЗ__ЎЈ
ЈЁ4Ј©ОӘ·АЦ№№эБҝөД°ұЖшУ°ПмПхЛбөДЦЖұёәНРФЦККөСйЈ¬ҝЙФЪ¶ЎЎўОмЦ®јдМнјУТ»ёцUРНёЙФп№ЬЈ¬ЖдЦРКўЧ°өДКФјБҝЙТФКЗПВБРЦРөД__ЎЈЈЁМоұкәЕЈ©
A.јоКҜ»Т
B.ЙъКҜ»Т
C.ЕЁБтЛб
D.ОЮЛ®ВИ»ҜёЖ
ЈЁ5Ј©ёДЧ°әуЈ¬КөСйЦР№ЫІмөҪОмЧ°ЦГЦРөДЖшМеұдОӘәмЧШЙ«Ј¬јәЖҝЦРИЬТәСХЙ«ұдәмЎЈИфНЁИлөҪјәЖҝЦРөДәмЧШЙ«»мәПЖшМеЗЎәГУлЛ®НкИ«·ҙУҰЗТОЮЖдЛыЖшМеЙъіЙЈ¬ФтјәЖҝЦР·ўЙъ·ҙУҰөД»ҜС§·ҪіМКҪОӘ___ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝФЪТ»¶ЁМхјюПВДіМе»эТ»¶ЁөДГЬұХИЭЖчЦР·ўЙъөД·ҙУҰaA(g)+bB(g)![]() xC(g)·ыәППВНјјЧЛщКҫ№ШПө(c%ұнКҫЖҪәв»мәПЖшМеЦРCөД°Щ·Цә¬БҝЈ¬TұнКҫОВ¶ИЈ¬pұнКҫС№Зҝ)ЎЈФтНјТТЦРЧЭЦбyКЗЦё
xC(g)·ыәППВНјјЧЛщКҫ№ШПө(c%ұнКҫЖҪәв»мәПЖшМеЦРCөД°Щ·Цә¬БҝЈ¬TұнКҫОВ¶ИЈ¬pұнКҫС№Зҝ)ЎЈФтНјТТЦРЧЭЦбyКЗЦё

A. ЖҪәв»мәПЖшөДГЬ¶И
B. ЖҪәв»мәПЖшЦРBөД°Щ·Цә¬Бҝ
C. ЖҪәв»мәПЖшөДЧЬОпЦКөДБҝ
D. ЖҪәв»мәПЖшөДЖҪҫщПа¶Ф·ЦЧУЦКБҝ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝТ»¶ЁМхјюПВҙжФЪ·ҙУҰЈәCO(g)Ј«H2O(g)![]() CO2(g)Ј«H2(g)Ј¬ЖдХэ·ҙУҰ·ЕИИЎЈПЦУРИэёцПаН¬өД2 LәгИЭҫшИИ(УлНвҪзГ»УРИИБҝҪ»»»)ГЬұХИЭЖчўсЎўўтЎўўуЈ¬ФЪўсЦРідИл1 mol COәН1 mol H2OЈ¬ФЪўтЦРідИл1 mol CO2әН1 mol H2Ј¬ФЪўуЦРідИл2 mol COәН2 mol H2O,700 ЎжМхјюПВҝӘКј·ҙУҰЎЈҙпөҪЖҪәвКұЈ¬ПВБРЛө·ЁХэИ·өДКЗ(ЎЎЎЎ)
CO2(g)Ј«H2(g)Ј¬ЖдХэ·ҙУҰ·ЕИИЎЈПЦУРИэёцПаН¬өД2 LәгИЭҫшИИ(УлНвҪзГ»УРИИБҝҪ»»»)ГЬұХИЭЖчўсЎўўтЎўўуЈ¬ФЪўсЦРідИл1 mol COәН1 mol H2OЈ¬ФЪўтЦРідИл1 mol CO2әН1 mol H2Ј¬ФЪўуЦРідИл2 mol COәН2 mol H2O,700 ЎжМхјюПВҝӘКј·ҙУҰЎЈҙпөҪЖҪәвКұЈ¬ПВБРЛө·ЁХэИ·өДКЗ(ЎЎЎЎ)
A.ИЭЖчўсЎўўтЦРХэ·ҙУҰЛЩВКПаН¬B.ИЭЖчўсЎўўуЦР·ҙУҰөДЖҪәвіЈКэПаН¬
C.ИЭЖчўсЦРCOөДОпЦКөДБҝұИИЭЖчўтЦРөДЙЩD.ИЭЖчўсЦРCOөДЧӘ»ҜВКУлИЭЖчўтЦРCO2өДЧӘ»ҜВКЦ®әНРЎУЪ1
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝКөСйРЎЧйАыУГNa2SO3әНЕЁБтЛбЦЖұёSO2ІўҪшРРПа№ШКөСйМҪҫҝЎЈКөСй·Ҫ°ёИзНјЎЈ
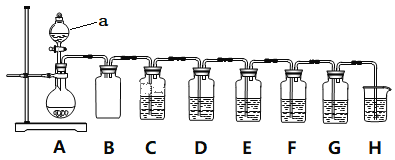
ТСЦӘЈәёчЧ°ЦГЦРөДКФјБЈ¬C(Ж·әмИЬТә)ЎўD(ЛбРФKMnO4ИЬТә)ЎўE(H2SИЬТә)ЎўF(өн·ЫI2Л®ИЬТә)ЎўG(H2O2әНBaCl2»мәПТә)
(1)ТЗЖчЧйЧ°НкәуКЧПИТӘҪшРРөДІЩЧчКЗ____________________ЎЈ
(2)ТЗЖчaөДГыіЖКЗ________________Ј»Ч°ЦГBөДЧчУГ_____________________ЎЈ
(3)Ч°ЦГAЦР·ўЙъ·ҙУҰөД»ҜС§·ҪіМКҪ____________________ЎЈ
(4)Ч°ЦГCәНGЦРөДПЦПуЈәC___________________Ј»G___________________Ј»
(5)ЙијЖЧ°ЦГDәНEөДДҝөДКЗСйЦӨSO2өД___________РФәН___________РФЎЈ
(6)Ч°ЦГHЦРөДКФјБКЗ______________Ј»ЖдЧчУГКЗ_____________________ЎЈ
(7)FЦР·ҙУҰөДАлЧУ·ҪіМКҪ____________________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝҪХёС(ә¬¶аМЗОпЦК)өДЧЫәПУҰУГҫЯУРЦШТӘөДТвТеЎЈПВГжКЗТФҪХёСОӘФӯБПәПіЙҫЫхҘАаёЯ·ЦЧУ»ҜәПОпөДВ·ПЯЈә
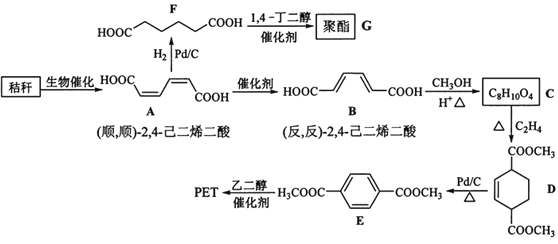
»ШҙрПВБРОКМвЈә
ЈЁ1Ј©ПВБР№ШУЪМЗАаөДЛө·ЁХэИ·өДКЗ___ЎЈ(МоұкәЕ)
a.МЗАа¶јУРМрО¶Ј¬ҫЯУРCnH2mOmөДНЁКҪ
b.ВуСҝМЗЛ®ҪвЙъіЙ»ҘОӘН¬·ЦТм№№МеөДЖПМСМЗәН№ыМЗ
c.УГТшҫө·ҙУҰІ»ДЬЕР¶Пөн·ЫЛ®ҪвКЗ·сНкИ«
d.өн·ЫәНПЛО¬ЛШ¶јКфУЪ¶аМЗАаМмИ»ёЯ·ЦЧУ»ҜәПОп
ЈЁ2Ј©BЙъіЙCөД·ҙУҰАаРНОӘ___ЎЈ
ЈЁ3Ј©DЦР№ЩДЬНЕГыіЖОӘ____Ј¬DЙъіЙEөД·ҙУҰАаРНОӘ___ЎЈ
ЈЁ4Ј©FөД»ҜС§ГыіЖКЗ___Ј¬УЙFЙъіЙGөД»ҜС§·ҪіМКҪОӘ___ЎЈ
ЈЁ5Ј©ҫЯУРТ»ЦЦ№ЩДЬНЕөД¶юИЎҙъ·јПг»ҜәПОпWКЗEөДН¬·ЦТм№№МеЈ¬0.5molWУлЧгБҝМјЛбЗвДЖИЬТә·ҙУҰЙъіЙ44gCO2Ј¬W№ІУР___ЦЦ(І»ә¬БўМеҪб№№)Ј¬ЖдЦРәЛҙЕ№ІХсЗвЖЧОӘИэЧй·еөДҪб№№јтКҪОӘ____ЎЈ
ЈЁ6Ј©ІОХХЙПКцәПіЙВ·ПЯЈ¬ТФ(·ҙЈ¬·ҙ)-2Ј¬4-јә¶юП©әНC2H4ОӘФӯБП(ОЮ»ъКФјБИОСЎ)Ј¬ЙијЖЦЖұё¶ФұҪ¶юјЧЛбөДәПіЙВ·ПЯ___ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com