
����Ŀ�������£���20 mL 0��2 mol��L-1��Ԫ��H2A��Һ�еμ�0��2 mol��L-l NaOH��Һ���й������ʵ����仯��ͼ������������ȷ���ǣ� ��
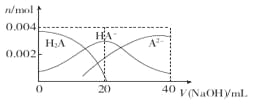
A. ��V��NaOH��="20" mLʱ����Һ�и�����Ũ�ȵĴ�С˳��Ϊ��c��Na+��>c��HA-��>c�� A2-��>c�� OH-��> ����H+��
B. �������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ�еĴ�
C. ��Ũ��H2A��NaHA�Ļ����Һ�����ۼ���������ǿ���ǿ���Һ��pH�仯������
D. ��V��NaOH�� ="40" mLʱ�������¶ȣ�c��Na+����c��A2-����С
���𰸡�C
��������
����A����V��NaOH��="20" mLʱ��0��2 mol��L-lNaOH��20 mL 0��2 mol��L-1H2A��Ӧ����NaHA������Ũ�ȴ�С��˳��Ϊc��Na+��>c��HA-��>c��OH-��> c��H+��> c�� A2-��������B���������Ũ�ȵ�NaOH��Һ��H2A��Һ�������NaHA����ͼ���֪��������20mLNaOH��Һʱ��A2-��Ũ�ȴ���H2A��˵��HA-�ĵ���̶ȴ���ˮ��̶ȣ��������ˮ�ĵ���̶ȣ�������Һ��ˮ�ĵ���̶ȱȴ�ˮ�е�С������C����Ũ��H2A��NaHA�Ļ����Һ�����ۼ���������ǿ���ǿ���Һ��pH�仯��������ȷ��D����V��NaOH�� ="40" mLʱ������Na2A��A2-�ᷢ��ˮ�⣬�����¶ȣ��ٽ�ˮ��̶ȣ���c��A2-����С����c��Na+��/c��A2-�����ʴ�ΪC��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���״���Ϊ�������л�������Ʒ�ͻ�������ȼ�Ͼ��й�����Ӧ��ǰ����CO2����ϳɼ״��Ǻ�������CO2����Ч;������CO2�Ʊ��״����̿����漰��Ӧ���£�
��Ӧ��CO2(g)��H2(g) ![]() CO(g)��H2O(g) ��H1����41.19 kJ��mol��1
CO(g)��H2O(g) ��H1����41.19 kJ��mol��1
��Ӧ��CO(g)��2H2(g) ![]() CH3OH(g) ��H2
CH3OH(g) ��H2
��Ӧ��CO2(g)��3H2g) ![]() CH3OH(g)��H2O(g) ��H3����49.58 kJ��mol��1
CH3OH(g)��H2O(g) ��H3����49.58 kJ��mol��1
�ش��������⣺
��1����Ӧ��Ļ�ѧƽ�ⳣ���ֱ�ΪK1��K2��K3����K3=__________����K�Ĵ���ʽ��ʾ��
��2���ں�ѹ�ܱ������У�����һ������ H2 �� CO2���ٶ���������Ӧ��ʵ���÷�Ӧ���ڲ�ͬ�¶��£���Ӧ��ϵ�� CO2 ��ƽ��ת������ѹǿ�Ĺ�ϵ������ͼ 1 ��ʾ��

�ٱȽ� T1 �� T2 �Ĵ�С��ϵ��T1____________T2���������������������
���� T1 �� p6 �������£����ܱ������г��� 3 mol H2 �� 1 mol CO2���÷�Ӧ�ڵ� 5 min ʱ�ﵽƽ�⣬��ʱ���������Ϊ 1.8 L����÷�Ӧ�ڴ��¶��µ�ƽ�ⳣ��Ϊ________��
a.���������·�Ӧ�� 3 min ʱ�̣��ı��������� A �㴦�ﵽƽ�⣬CH3OH ��Ũ���淴Ӧʱ��ı仯������ͼ 2��ʾ��3��4 min ��Ũ�ȱ仯δ��ʾ����������ı������Ϊ __________������ H2 ��Ũ�ȱ仯�����4 min��ʼ�� A��ķ�Ӧ����v(H2)= _________(������λС��)��
b.���¶Ȳ��䣬ѹǿ�㶨�� p8 �����������´ﵽƽ��ʱ�������������Ϊ_______L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ϊ�����Դ���Ź㷺��Ӧ��ǰ����������Ȼ���Ʊ��������������¡�

��ش��������⣺
I��ת��������Ȼ��ѹ����������30��ʱ����T��F�������£����Ի������������ʾ��ͼ���¡�
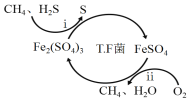
(1)����i�����ӷ�Ӧ����ʽΪ_________________________________________��
(2)��֪��
��Fe3+��pH=l.9ʱ��ʼ������pH=3.2ʱ������ȫ��
��30��ʱ����T.F�������£���ͬpH��FeSO4��Һ��Fe2+�������������±���
pH | 0.7 | 1.1 | 1.5 | 1.9 | 2.3 | 2.7 |
Fe2+����������/g��L-1��h-1 | 4.5 | 5.3 | 6.2 | 6.8 | 7.0 | 6.6 |
��ת�������У������ϱ���ѡ�����pH��Χ��_______<pH<_______������ѡ���ԭ���ǣ�_______________________________________________��
������ת�����ڴ����������£�ˮ������CH4�����������ͼ�ش����⡣
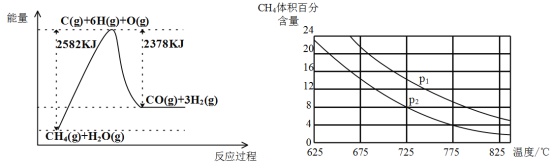
(3)�ٸù��̵��Ȼ�ѧ����ʽ��__________________________________________��
�ڱȽ�ѹǿP1��p2�Ĵ�С��ϵ��P1 _________ P2(ѡ����>����<������=��)��
����һ���¶Ⱥ�һ��ѹǿ�µ�����ɱ���ܱ������г���1molCH4��1mol��ˮ������ַ�Ӧ��ƽ������ʼʱ��������ܶ���ƽ��ʱ������ܶȵ�1.4��������ʱ���������Ϊ2L,��÷�Ӧ��ƽ�ⳣ��Ϊ______________(�������2λ��Ч����)��
����CO�任��500��ʱ��CO��һ����ˮ��Ӧ����CO2��H2��
����H2�ᴿ����CO2��H2����õ�H2�Ĺ�����ʾ��ͼ
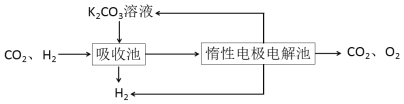
(4)���ճ��з�����Ӧ�����ӷ���ʽ��____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�������ͬ���칹����Ŀ���ٵ���
A. ����B. C5H11Cl
C. ��ϩD. �������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������£����ݻ�Ϊ2L�ĺ����ܱ������У���2molN�����3molM�������ϣ��������·�Ӧ��2N(g)+3M(g)![]() xQ(g)+3R(g)��4s��÷�Ӧ��ƽ��ʱ������2.4molR�������Q�ķ�Ӧ����Ϊ0.1mol/��L��s���������й�������ȷ����
xQ(g)+3R(g)��4s��÷�Ӧ��ƽ��ʱ������2.4molR�������Q�ķ�Ӧ����Ϊ0.1mol/��L��s���������й�������ȷ����
A. N��ת����Ϊ80% B. 0��4s�ڣ���������ƽ����Է�����������
C. xֵΪ2 D. ƽ��ʱM��Ũ��Ϊ0.6mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ǰ�����ڵ�A��B��C��D����Ԫ�������ڱ��о���Ԫ��X�������ڡ���֪Ԫ��X���������Ļ�ѧʽΪX2O5��B��Dͬ������BԪ�ص�ԭ�Ӱ뾶��ͬ��Ԫ������С�ģ�C������������Ӧ��ˮ������ǿ�ᡣ
��1��DԪ�ػ�̬ԭ�ӵ���Χ�����Ų�ʽΪ____________________��
��2��A��C��X����Ԫ��ԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ________________������Ӧ��Ԫ�ط������𣩡�
��3��B��X��D�⻯��ķе��ɸߵ��͵�˳��Ϊ_______________������Ӧ�Ļ�ѧʽ���𣩡�
��4��CԪ�ص�ԭ�ӿ��γɶ������ӣ����Ʋ������������幹�ͣ�CΪ��ĸ������̼Ԫ�أ���
�� | CO32- | CO42- |
���幹������ | _______________ | _______________ |
��5��Ԫ��B��һ���⻯��B2H4������Ҫ����;���й�B2H4��˵����ȷ����_______��
A��B2H4���Ӽ���γ���� B��Bԭ����sp3�ӻ�
C��B2H4�����к���5���Ҽ���1���м� D��B2H4�����ΪҺ̬ʱ�ƻ����ۼ�
��6��EԪ�غ�DԪ����ͬһ���ڣ�����VIII�壬�۲������������ӣ�E(OH)2Ϊ�������������Ũ��ǿ����Һ�п��γ�E(OH)42-��д��E(OH)2��ʽ����ĵ��뷽��ʽ___________________��
��7��FԪ�ػ�̬ԭ��M������5�ԳɶԵ��ӣ�F�γɵĵ����Цġ��á�������ͬ�������壬���־�������ͼ��ʾ����Fԭ�ӵ���λ��֮��Ϊ___________���ġ��á������־����ı߳�֮��Ϊ_____________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ������������й�˵����ȷ����
A. ���³�ѹ�£�1.8g��(��CD3)�к��е�������ΪNA
B. ��״���£�11.2L��ϩ�ͻ�����(C3H6)�Ļ�������У����õ��ӶԵ���ĿΪ3NA
C. ����ͭ�뺬0.4 mol HNO3��Ũ���ᷴӦ������ת��������0.2NA
D. �����£�1L pH=9��CH3COONa��Һ�У����������ˮ������Ϊ1��10��9 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ϊ98%�ܶ�Ϊ1.84g/cm3��ŨH2SO4���Ƴ�500ml0.5mol/L��ϡH2SO4�����㣺
��1��ŨH2SO4�����ʵ���Ũ���Ƕ��٣�_____
��2������ŨH2SO4������Ƕ��٣�_____(д����ع�ʽ���������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�ⶨij���ᾧ����H2C2O42H2O����Ʒ�Ĵ������ֳ�ȡһ�������ĸ���Ʒ�����Ƴ�100 mL��Һ��ȡ25.00 mL����Һ����ƿ����������ϡ��������0.100 mol/L��KMnO4��Һ�ζ�(���ʲ����뷴Ӧ)��Ϊʡȥ������̣�����ȡ����Ʒ������Ϊij��ֵʱ���ζ�����KMnO4��Һ�ĺ�����ǡ�õ�����Ʒ�в��ᾧ�������������100������Ӧ��ȡ��Ʒ������Ϊ
A. 2.25 g B. 3.15 g C. 9.00 g D. 12.6 g
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com