
һ��ⶨ��Ʒ�гɷֺ�����ʵ��Ӧ�ظ�2��3�Ρ�Ϊ�˲ⶨij�������ƹ����л��е�̼���Ƶ������������ס��ҡ�����λͬѧ�ֱ����������ʵ�鷽����

��ͬѧ�ķ�����ͼ��ʾ��
��1����μ���Aװ�õ������ԣ�_____________________________________________��
��2����ͬѧ�ظ�����������ʵ�飬�õ�̼���Ƶ��������������ݴ��ڽϴ��ƫ�����Ϊ��������������ƫ�͵�ԭ����_______������ţ���
A��װ����ԭ�п����еĶ�����̼����Ҳ����ʯ������
B��װ��������е�ˮ�����Ͷ�����̼����ʯ������
C����Ӧ��ɺ�װ���еĶ�����̼û��ȫ������ʯ������
D������ϡ����������㡢��Ӧ�����
��3��Ϊ���ü�ʵ����������ȷ��������ʵ�鲽�趼��ȷ�������£�����Ϊͼ�е�ʵ��װ��Ӧ����θĽ���______________��
����ͬѧ�ķ����ǣ���ͼ�����ṩ��װ����ѡ��ʵ��װ�ã������ͬѧʵ��װ���е�B��C��ͨ���ⶨ�ų��Ķ�����̼������������Ƕ�����̼����ˮ�������㡣

ѡ�����װ�õ�����˳��Ϊ_______��
��ͬѧ�ķ����ǣ���ȡ��Ʒm g�����ܽ⣬��������Ȼ�����Һ�����ˡ�ϴ�ӡ���ɡ��������ù���n g��
��1������100 mL 0��10 mol/L BaCl2��Һ��ʵ��������IJ����������ձ�������������ͷ�ιܡ���Ͳ���_______�����������ƣ���
��2���������̼���Ƶ���������Ϊ����m��n��ʾ��_______��
��3��Ca2+��Ba2+������ʹ ������ȫ���ܷ�ʹ���Ȼ�����Һ�����Ȼ�����Һ��_______ ����ܡ�����ԭ���ǣ�_____________________________________��
������ȫ���ܷ�ʹ���Ȼ�����Һ�����Ȼ�����Һ��_______ ����ܡ�����ԭ���ǣ�_____________________________________��
��1����ֹˮ�мн�Aװ�õ�����ĩ�˵���Ƥ�ܣ���Һ©���ϲ������ӣ�������ע����������ˮ����������ʼ������ˮ���£���һ�����ˮ���ܵ���Բ����ƿ��֤��װ������������
��2��C��D ��3����װ��ʯ�ҵĸ�����ұ���װһ��ʢ�м�ʯ�ҵĸ���ܣ���ֹ������ˮ�ֺͶ�����̼��Cװ���еļ�ʯ������ �ݢߢ�
��1��100 mL����ƿ
��2��106n/197m
��3���� ������Ca2+����OH-��������ˮ���������Ƴ�����Ӱ����
����������1������Aװ�������Եķ�������ֹˮ�мн�Aװ�õ�����ĩ�˵���Ƥ�ܣ���Һ©���ϲ������ӣ�������ע����������ˮ����������ʼ������ˮ���£���һ�����ˮ���ܵ���Բ����ƿ��֤��װ�����������á���2����װ����ԭ�п����е�CO2���屻��ʯ������ʱ�����CO2�����������²������ƫ�ߣ���װ��������е�ˮ������CO2����ʯ������ʱ�����CO2�����������²������ƫ�ߣ���װ���еĶ�����̼û��ȫ������ʯ������ʱ�����CO2���������٣����²������ƫ�ͣ�������ϡ����������㣬��Ӧ�����ʱ��������CO2���٣����CO2���������٣����²������ƫ�͡���3��Ϊ���ü�ʵ����������ȷ��Ӧ��װ��ʯ�ҵĸ�����ұ���װһ��ʢ�м�ʯ�ҵĸ���ܣ���ֹ������ˮ�ֺͶ�����̼��Cװ���еļ�ʯ�����ա�
����������װ��֪���ⶨCO2��������Ӧ�á���ˮ��������������ѡ��װ�âݡ��ޡ��ߣ������d�ڽ�����c�ڳ�ˮ��ˮ��װ�â߽���װ�âޡ���1������һ�����ʵ���Ũ�ȵ���Һ���õ��IJ��������У��ձ�����������100 mL����ƿ����ͷ�ιܡ���Ͳ����2��n��Na2CO3��=n��BaCO3��= =
=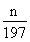 mol����Na2CO3������������
mol����Na2CO3������������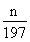 mol��106 g/mol��m g=106n/197m��
mol��106 g/mol��m g=106n/197m��
��3�����ڹ�����Ca2+����OH-��������ˮ���������Ƴ�����Ӱ�������������Բ���ʹ���Ȼ�����Һ�����Ȼ�����Һ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 4-4��������Ҫ��������ϰ���������棩 ���ͣ�ѡ����
�����й�˵����ʽ��ȷ���ǣ� ��
A.�ô�����������β���е�CO��NO��CO��NO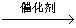 C��NO2
C��NO2
B. NO2��ˮ�ķ�Ӧ��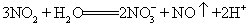
C. NH4HCO3���ڹ�����NaOH��Һ�У�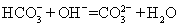
D.��1.0 mol��L-1��KNO3��Һ�У�H+��Fe2+��Cl-��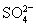 һ���ܴ�������
һ���ܴ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 4-1���ǽ������ϵ�����-����ϰ���������棩 ���ͣ�ѡ����
������������̼�����Ʒ�ĩ���Ƿ����̼���Ƶ�ʵ�鷽���ǣ� ��
A�����ȣ�����������ų�
B���μ����ᣬ����������ų�
C������ˮ�μ�ϡ���Ȼ�����Һ�����ް�ɫ��������
D������ˮ�μӳ���ʯ��ˮ�����ް�ɫ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 3-2��������Ҫ��������ϰ���������棩 ���ͣ������
���ڽ������ӵ�ij�ֺ�������ҩƤ�ɴ���ʯ��ˮ�ࡢ���������ƶ��ɡ�
��1��Al��ԭ�ӽṹʾ��ͼΪ____________��Al��NaOH��Һ��Ӧ�����ӷ���ʽΪ____________��
��2��30Si��ԭ�ӵ�������Ϊ________________________��
��3��Al3+��Yn-�ĵ�������ͬ��Y�������Ԫ�ص��⻯���ˮ��Һ�������ԣ�������⻯���зе���͵���____________________________________��
��4�����ӹ����У�ҩƤ�ڸ����²�����������ʹ�����������������壬��������____________��
��5���������������36.0 g������Fe2O3��Al2O3��SiO2������������ϡ���ᣬ����õ�11.0 g���壻��Һ�м������NaOH��Һ������õ�21.4 g���壻���������Al2O3����������Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 3-2��������Ҫ��������ϰ���������棩 ���ͣ�ѡ����
�������ʵ�����þ�������Ȼ�ϣ�ȡ�������û�����ķݣ��ֱ�ӵ�������������Һ�У���ַ�Ӧ�ų����������ǣ� ��
A.3 mol��L-1 ����
B.4 mol��L-1 HNO3��Һ
C.5 mol��L-1 NaOH��Һ
D.18.4 mol��L-1 H2SO4��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 3-1�Ƽ�����Ҫ��������ϰ���������棩 ���ͣ�ѡ����
��8 g Na2O2��Na2O��Na2CO3��NaOH�Ļ������200 g��������Ϊ3��65%������ǡ�÷�Ӧ��������Һ�����յù�������Ϊ�� ��
A��8 g B��15��5 g C��11��7 g D��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 3-1�Ƽ�����Ҫ��������ϰ���������棩 ���ͣ�ѡ����
����������ȷ���ǣ� ��
A����Ũ���ᱣ������ɫ����ƿ��
B�������ƺͼر�����ú����
C��Na2CO3���Ա����ڲ������IJ���ƿ��
D��NaOH���������ֽ�ϳ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 2-2���ӷ�Ӧ��ϰ���������棩 ���ͣ�ѡ����
����A��D���飬ÿ����������Ӧ������������Ӧ����ͬһ�����ӷ���ʽ��ʾ���ǣ� ��
��������
A����SO2ͨ��Ba��OH��2��Һ������SO2ͨ������Ba��OH��2��Һ��
B����Ũ��ˮ����Al2��SO4��3��Һ������Al2��SO4��3��Һ����Ũ��ˮ��
C0.1 mol Cl2ͨ�뺬0.2 mol FeBr2����Һ��0.3 mol Cl2ͨ�뺬0.2 mol FeBr2����Һ��
D����BaCl2��Һ������Na2SO4��Һ��������Ba��OH��2��Һ�����MgSO4��Һ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 11-3���ʵ��Ʊ���ϰ���������棩 ���ͣ�ѡ����
�����ͼ���������������У���ȷ����( )

A��ͼ1��ʾװ�ÿ����ڷֱ���ȡ����NO��H2
B��ͼ1��ʾװ�ÿ�������֤�����ԣ�KMnO4��Cl2
C��ϴ�ӳ���ʱ(��ͼ2)����©���м�����ˮ�����貢�˸�
D������ͼ2��ʾ�ķ��������ᴿ����������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com