
����Ŀ�������İ���ͭ����([Cu(NH3)4]SO4��H2O)������ɱ�����ýȾ�����ڼ��Զ�ͭ��Ҳ���������Һ����Ҫ�ɷ֣��ڹ�ҵ����;�㷺�������¸������ڿ����в��ȶ�������ʱ�����ֽ⡣ij��ѧ��ȤС����Cu�ۡ�3mol/L�����ᡢŨ��ˮ��10% NaOH��Һ��95%���Ҵ���Һ��0.500 mol/Lϡ���ᡢ0.500 mol/L��NaOH��Һ���ϳ������İ���ͭ���岢�ⶨ�䴿�ȡ�
I��CuSO4��Һ���Ʊ�
�ٳ�ȡ4gͭ�ۣ���A����������10���Ӳ����Ͻ��裬������ȴ��
�����������м���30mL 3mol/L�����ᣬ��A�й��������������У����Ȳ����Ͻ��衣
�۳��ȹ��˵���ɫ��Һ��
(1)A����������Ϊ________________________________��
(2)ijͬѧ��ʵ������1.5g��ͭ��ʣ�࣬��ͬѧ���Ƶõ�CuSO4��Һ������һ�������м���Ũ�����о�Ĥ���֣���ȴ�����ľ����к��а�ɫ��ĩ���Խ�����ԭ��__________________________________________��
II��������Ʊ�
�������Ʊ���CuSO4��Һ����ͼ��ʾ���в���
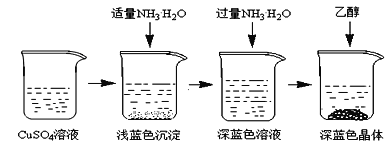
(3)��֪dz��ɫ�����ijɷ�ΪCu2(OH)2SO4����д�����ɴ˳��������ӷ�Ӧ����ʽ___________________��
(4)��������ʱ���ü����Ҵ��ķ�����������Ũ���ᾧ��ԭ����________________________��
III���������IJⶨ
��ȷ��ȡwg������������ˮ�ܽ���ע����ͼ��ʾ������ƿ����Ȼ����μ�������10%NaOH��Һ��ͨ��ˮ����������ƷҺ�еİ�ȫ����������������ˮ��ϴ�����ڱڣ���V1mL0.5mol/L���������Һ��ȫ���ա�ȡ�½���ƿ����0.5mol/L NaOH����Һ�ζ���ʣ��HCl(ѡ�ü�����ָʾ��)�����յ�ʱ����V2mLNaOH��Һ��
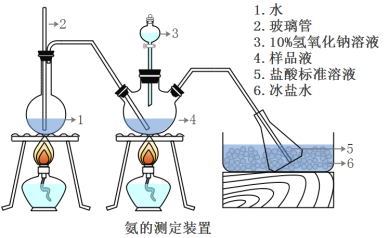
(5)Aװ���г������ܵ�����_________________����Ʒ�а������������ı���ʽ_______��
(6)����ʵ���������ʹ�������ⶨ���ƫ�ߵ�ԭ����____________________��
A���ζ�ʱδ��NaOH����Һ��ϴ�ζ��ܡ�
B������ʱ���ζ�ǰƽ�ӣ��ζ����ӡ�
C���ζ�������ѡ�÷�̪��ָʾ����
D��ȡ�½���ƿǰ��δ������ˮ��ϴ�������ƿ�еĵ�����ڡ�
���𰸡����� ��Ӧ�������������Ũ�������У�ϡ������Ũ��Ũ�������ˮ��ʹCuSO4![]() 5H2Oʧˮ���CuSO4 2Cu2++2NH3
5H2Oʧˮ���CuSO4 2Cu2++2NH3![]() H2O+SO42-=Cu2(OH)2SO4+2NH4+ �����İ���ͭ�����������ȷֽ� ƽ����ѹ����ֹ�����͵���
H2O+SO42-=Cu2(OH)2SO4+2NH4+ �����İ���ͭ�����������ȷֽ� ƽ����ѹ����ֹ�����͵��� ![]() BD
BD
��������
(1) ��������һ���������н�����
(2) ���Ƶõ�CuSO4��Һ������һ�������м���Ũ�����о�Ĥ���֣���ȴ�����ľ����к��а�ɫ��ĩ���ð�ɫ��ĩΪCuSO4��ԭ���Ƿ�Ӧ�������������Ũ�������У�ϡ������Ũ��Ũ�������ˮ��ʹCuSO4![]() 5H2Oʧˮ���CuSO4��
5H2Oʧˮ���CuSO4��
(3)�ɲ������̿�֪������ͭ��Һ��������ˮ��Ӧ����Cu2(OH)2SO4�������ݴ�д�����ӷ���ʽ��
(4) ������狀�ͭ������Ʊ������У������Ҵ����Խ��������İ���ͭ������ܽ�ȣ������ھ���������
(5) ��A��ѹ������ʱ����������Һ��������ʹAƿ��ѹ���ȶ���
���ݵζ���ȥ���������Ƶ����ʵ������Լ�����백��Ӧ����������ʵ�����������������İ��������ʵ������ټ����NH3�������ٷ�����
(6) ������Ʒ�а���������������ʽ![]() ������������V2ƫС����ʹ�������ⶨ���ƫ�ߣ��ݴ˷�����
������������V2ƫС����ʹ�������ⶨ���ƫ�ߣ��ݴ˷�����
(1) ��������һ���������н��У���ͭ��ת��ΪCuO��
�ʴ�Ϊ��������
(2) ���Ƶõ�CuSO4��Һ������һ�������м���Ũ�����о�Ĥ���֣���ȴ�����ľ����к��а�ɫ��ĩ���ð�ɫ��ĩΪCuSO4��ԭ���Ƿ�Ӧ�������������Ũ�������У�ϡ������Ũ��Ũ�������ˮ��ʹCuSO4![]() 5H2Oʧˮ���CuSO4��
5H2Oʧˮ���CuSO4��
�ʴ�Ϊ����Ӧ�������������Ũ�������У�ϡ������Ũ��Ũ�������ˮ��ʹCuSO4![]() 5H2Oʧˮ���CuSO4��
5H2Oʧˮ���CuSO4��
(3)�ɲ������̿�֪������ͭ��Һ��������ˮ��Ӧ����Cu2(OH)2SO4���������ӷ�Ӧ����ʽΪ��2Cu2++2NH3![]() H2O+SO42-=Cu2(OH)2SO4+2NH4+��
H2O+SO42-=Cu2(OH)2SO4+2NH4+��
�ʴ�Ϊ��2Cu2++2NH3![]() H2O+SO42-=Cu2(OH)2SO4+2NH4+��
H2O+SO42-=Cu2(OH)2SO4+2NH4+��
(4) �������Ϣ��֪�������İ���ͭ�����������ȷֽ⣬�������Ҵ����Խ��������İ���ͭ������ܽ�ȣ������ھ���������
�ʴ�Ϊ�������İ���ͭ�����������ȷֽ���
(5) ��A��ѹ������ʱ������������Һ��������ʹAƿ��ѹ���ȶ������������ܵ�������ƽ����ѹ����ֹ�����͵�����
�ʴ�Ϊ��ƽ����ѹ����ֹ�����͵�����
��������ݿ�֪�������İ��������ʵ���Ϊ(0.5V1-0.5V2)��10-3mol��
������Ʒ�а�����������Ϊ��![]() ��
��
�ʴ�Ϊ��![]() ��
��
(6)������Ʒ�а���������������ʽ![]() ����ʵ���������V2ƫС����ʹ�������ⶨ���ƫ�ߣ�
����ʵ���������V2ƫС����ʹ�������ⶨ���ƫ�ߣ�
A���ζ�ʱδ��NaOH����Һ��ϴ�ζ��ܣ�����������ҺŨ��ƫС�����ĵ����V2ƫ����ʹ�������ⶨ���ƫ�ͣ����������⣻
B������ʱ���ζ�ǰƽ�ӣ��ζ����ӣ����ĵ����V2ƫС����ʹ�������ⶨ���ƫ�ߣ��������⣻
C���ζ�������ѡ�÷�̪��ָʾ�������ĵ����V2ƫ����ʹ�������ⶨ���ƫ�ͣ����������⣻
D��ȡ�½���ƿǰ��δ������ˮ��ϴ�������ƿ�еĵ�����ڣ����ĵ����V2ƫС����ʹ�������ⶨ���ƫ�ߣ��������⡣
�ʴ�Ϊ��BD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ� ��
A. ���ӻ�����һ�����ɽ�����ǽ���Ԫ����ɵ�
B. ���ۻ������п��ܺ������Ӽ�
C. ���ӻ������п��ܺ��й��ۼ�
D. ֻ���й��ۼ��������ǹ��ۻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʹ�������Դ��չ����̼����������Ϊ��ѧ���о�����Ҫ���⡣�������״������ʵ����ȼ�ϣ�������ȼ�ϵ�ء�
��1����֪����2CH3OH(l)+3O2(g)=2CO2(g)+4H2O(g) ��H1= -1275.6 kJ��mol-1����2CO(g)+O2(g)=2CO2(g) ��H2=-566.0 kJ��mol-1����H2O(g)=H2O(l) ��H3= -44.0 kJ��mol-1��
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��_______________��
��2����ҵ��һ��ɲ������·�Ӧ���ϳɼ״���2H2(g)+CO(g)![]() CH3OH(g) ��H= -90.8kJmol-1��
CH3OH(g) ��H= -90.8kJmol-1��
��ij�¶��£���2mol CO��6mol H2����2L���ܱ������У���ַ�Ӧ10min�ﵽƽ��ʱ���c(CO)=0.2mol/L����CO��ת����Ϊ____����CH3OH��ʾ�ù��̵ķ�Ӧ����v(CH3OH)=______��
��Ҫ��߷�Ӧ2H2(g)+CO(g)CH3OH(g)��CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��_______��
a������ b��������� c������CO��Ũ�� d������H2 e������������� f��������״�
��3����ͼ��һ����ѧ���̵�ʾ��ͼ��

��ͼ�м׳���________װ��(��������������ԭ�����)������OH������________��(����CH3OH������O2��)��
��д��ͨ��CH3OH�ĵ缫�ĵ缫��Ӧʽ�� ____________________________��
���ҳ����ܷ�Ӧ�����ӷ���ʽΪ______________________________________��
�ܵ��ҳ���B(Ag)������������5.40gʱ����ʱ����ij�缫����1.60gij����������е�ij����Һ������________(�����)��
A��MgSO4 B��CuSO4 C��NaCl D��Al(NO3)3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��������ȷ����

A. ͼ�ٿɱ�ʾ������ͨ�������Һ��������������Һ�����Եı仯
B. ����ͼ�ڿ��жϿ��淴ӦA2(g)+3B2(g)![]() 2AB3(g)����H��0
2AB3(g)����H��0
C. ͼ�۱�ʾ���������pH�������������Һ�ֱ���������п�۷�����Ӧ�������������(V)��ʱ�ı仯��ʾ��ͼ
D. ͼ�ܿɱ�ʾѹǿ�Կ��淴ӦA(g)+B(g)![]() 2C(g)+D(g)��Ӱ�죬�ҵ�ѹǿ��
2C(g)+D(g)��Ӱ�죬�ҵ�ѹǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ���������ʵĵ���ƽ�ⳣ�������ʾ��
��ѧʽ | CH3COOH | H2CO3 | HC1O |
����ƽ�ⳣ�� | 1.7��10-5 | K1=4.3��10-7 K2=5.6��10-11 | 3.0��10-8 |
��ش��������⣺
��1��CH3COOH��H2CO3��HC1O��������ǿ������˳��Ϊ______________________��
��2��������0.1 mol��L-1��CH3COOH��Һ�������{�¶ȣ�����4�ֱ���ʽ�������������______��
A��c(H+) B��c(H+)/c(CH3COOH) C�� c(H+)��c(OH-) D��c(CH3COO��)��c(H+)/c(CH3COOH)
��3��ȡ0.10mol CH3COOH �������ᣩ��������ʵ�飬����䵼����������ˮ���仯��ͼ��ʾ���Ƚ�a��b���������ʣ��>����<����=������

n(H+)��a_____b��c(CH3COO-)��a_____b����ȫ�к�ʱ����NaOH�����ʵ�����a_____b��
��4��H+Ũ����ͬ�������������ҺA(���ᣩ��B(CH3COOH)�քe��п�۷�Ӧ����������һ����Һ�д���п���ų�������������ͬ��������˵����ȷ����__________����д��ţ�
�ٷ�Ӧ����Ҫ��ʱ��B>A �ڿ�ʼ��Ӧʱ������A>B
�۲μӷ�Ӧ��п�����ʵ���A=B ��A����пʣ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ����ʵ����������Ӧ������ȷ����
ѡ�� | ʵ����ʵ | ���� |
A | ����������ͬ��Na2S2O3��H2SO4��Ӧʱc(Na2S2O3)Խ������S������ʱ��Խ�� | ��������������ʱ������Ӧ��Ũ�ȣ���ѧ��Ӧ���ʼӿ� |
B | �ڻ�ѧ��Ӧǰ�����������ͻ�ѧ���ʶ�û�з����ı� | ����һ�������뻯ѧ��Ӧ |
C | ���ʵ���Ũ����ͬ������ʹ���ֱ���������ġ���״��ͬ��п����Ӧ | ��Ӧ��ʼʱ������ͬ |
D | ���ݻ��ɱ���ܱ������з�����ӦH2(g)��I2(g) | ����Ӧ���ʼӿ죬�淴Ӧ���ʲ��� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��±�ؼ��仯����㷺��������Ȼ���С��ش��������⣺
(1)����(COCl2)��һ����Ҫ���л��м��壬��ũҩ��ҽҩ���������ϡ��۰��������Լ������϶���������;�������ķ������幹��Ϊ______________��������Ԫ�صĵ縺����С�����˳��Ϊ______________����COCl2��Ϊ�ȵ�����ķ��Ӻ�����(��дһ��)__________��
(2)�ճ������У�����������ɼ��⣬����ƣ��Դ�ԭ�ӽṹ�ǶȽ�����һ����____________________________��
(3)���־�̬��������ͷǾ�̬����������ɿ��Ŀ�ѧ������_____________��
(4)һ��ͭ���廯�ᄃ���ṹ��ͼ��ʾ������ͼ�е�Cuȥ�����ٰ����е�Br����Cu���õ�����ͭ�ľ����ṹ������ͭ�Ķѻ���ʽΪ___________��ijͬѧ����̬ͭԭ�Ӽ۵��Ӵ����дΪ3d94s2��Υ���˺�������Ų������е�______��

(5)���й�������ͭ���廯�ᄃ���ṹ˵����ȷ����_________(ѡ����ĸ���)��
A���û�����Ļ�ѧʽΪCuBr2
B��ͭ����λ��Ϊ8
C����ÿ��Br���ڵ�Br��12��
D����ͼ��P���Q���ԭ�����������ȷ��R���ԭ���������Ϊ(1/4,1/4,1/4)
(6)��ͼ��P���R���ԭ�Ӻ˼��Ϊa cm��NAΪ����٤��������ֵ����þ����ܶ�Ϊ______g/cm3��(�г�����ʽ����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��pKa=-lgKa��25��ʱ��H2SO3��pKa1=1.85��pKa2=7.19����0.1 mol��L-1NaOH��Һ�ζ�20mL 0.1mol��L-1H2SO3��Һ�ĵζ���������ͼ��ʾ(�����ϵ�����ΪpH)������˵����ȷ����

A. a��������Һ�У�2c(HSO3-)+c(SO32-)=0.1mol/L
B. b��������Һ�У�c(H+)+c(SO32-)=c(OH-)+c(H2SO3)
C. c��������Һ�У�c(Na+)>3c(HSO3-)
D. e��������Һ�У�c(Na+)> c(SO32-)> c(H+)> c(OH-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ë�����е��ص��ǣ� ��
A.��Ũ���ᶼ�Ի�ɫ
B.���պ����ս���ë����ζ
C.ȼ�ղ���ֻ�ж�����̼��ˮ
D.��һ���������ܷ���ˮ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com