
| ��һ�εζ� | �ڶ��εζ� | �����εζ� | |
| ������Һ�����mL�� | 25.00 | 25.00 | 25.00 |
| ����Һ�����mL�� | 9.99 | 10.01 | 10.60 |
| c(��)?V(�ζ�) |
| V(��) |
| 5 |
| 2 |
| 1 |
| 18 |
| 1.260g |
| 0.0100mol |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| A���ó����ʯ��ˮ�ɼ���NaHCO3��Na2CO3 |
| B����ȡNaHCO3�ķ�Ӧ���������ܽ��С��NaCl |
| C���ڵڢۡ��ܡ��ݲ����У���Ԫ�ؾ������� |
| D����ҵ��ͨ����ⱥ��MgCl2��Һ��ȡ����þ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
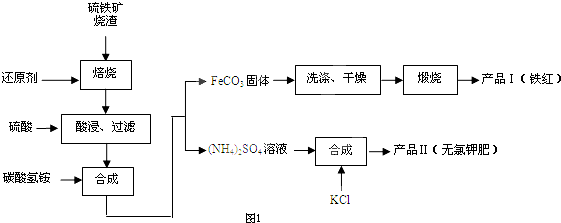
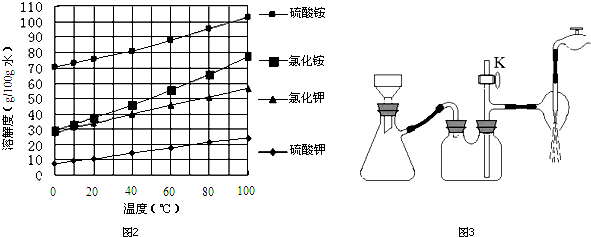
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| A����t1=15s��������C�� t0��t1ʱ��ε�ƽ����Ӧ����Ϊ0.004 mol?L-1?s-1 |
| B��t4��t5�θı������Ϊ��Сѹǿ��t5��t6�θı������������ѧ��Ӧ���¶� |
| C��B����ʼ���ʵ���Ϊ0.02 mol |
| D���û�ѧ��Ӧ�ı���ʽΪ��3A��g��?B��g��+2C��g�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
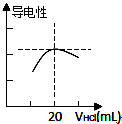 ijѧϰС�������кͷ�Ӧԭ���ⶨij��ˮ�����ʵ���Ũ�ȣ��Բ�����Һ�������жϵζ��յ㣮ʵ�鲽�����£�
ijѧϰС�������кͷ�Ӧԭ���ⶨij��ˮ�����ʵ���Ũ�ȣ��Բ�����Һ�������жϵζ��յ㣮ʵ�鲽�����£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����c1=c2�����Һ��c��NH4+��=c��Cl-������V1��V2 |
| B���������Һ��pH=7����c1V1��c2V2 |
| C����V1��V2��c1=c2������Һ��pH��7 |
| D���������Һ��pH��7������Һ��c��NH4+����c��OH-����c��Cl-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ��Ӧʱ��/min ���ʵ���Ũ��/mol?L-1 | 0 | 3 | 10 | 12 |
| C��H2�� | 2 | |||
| C��CO�� | 0.500 | 0.250 | 0.250 | |
| C��CH3OH�� | 0 | 0.500 | 0.750 | 0.750 |
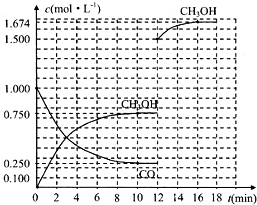
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��
| ||
B��
| ||
C��
| ||
D��
|
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com