
����Ŀ��a��b��c��d ��������֮���ת����ϵ��ͼ��ʾ(���ֲ�����ȥ)������˵����ȷ����

A. �� a Ϊ Cl2��b ����Ϊ NH3��ʵ���ҿ��ü��ȹ��� c �ķ�����ȡ NH3
B. �� a Ϊ Fe��b ����Ϊϡ HNO3����Ӧ�������ӷ���ʽΪ3Fe��8H+��2NO3-===3Fe2+��2NO2����4H2O
C. �� a Ϊ AlCl3 ��Һ��b ����Ϊ��ˮ����Ӧ�������ӷ���ʽΪAl3+��4NH3 ��H2O===AlO2-��4NH4+��2H2O
D. �� a Ϊ NaOH ��Һ��b ����Ϊ CO2������ Ca(OH)2 ��Һ���� c��d ��Һ�е�������
���𰸡�B
��������
A.��aΪCl2��b����ΪNH3������cΪ�Ȼ�泥����ڰ������Ȼ��ⷴӦ����ͨ�������Ȼ�淋ķ�����ȡNH3����A����
B.��aΪFe��b����ΪϡHNO3����Ӧ����������������Ӧ�����ӷ���ʽΪ��3Fe+8H++2NO3-�T3Fe2++2NO��+4H2O����B��ȷ��
C.��aΪAlCl3��Һ��b����Ϊ��ˮ���Ȼ����백ˮ��Ӧ��������������������ȷ�����ӷ���ʽΪ��Al3++3NH3H2O=Al��OH��3��+3NH4+����C����
D.��aΪNaOH��Һ��b����ΪCO2��cΪ̼�����ƣ�dΪ̼���ƣ�̼���ƺ�̼�����ƶ�������������Һ��Ӧ����̼��Ƴ���������Ca��OH��2��Һ��������D����
��ѡB��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������л�Ϊͬϵ�����( )
A. CH3��CH2��CH3��CH3��CH2��CH2��CH2��CH3B. CH3��CH3��CH3��CH![]() CH2
CH2
C. CH3��CH2��CH3��CH3��CH![]() CH2D. CH3��CH2��CH
CH2D. CH3��CH2��CH![]() CH2 ��CH3��CH2��CH2��CH3
CH2 ��CH3��CH2��CH2��CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������У����÷�Һ©�����з������
A.����CCl4B.���CCl4C.�ƾ���ˮD.���ͺ�ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����״���£�1.68L��ɫ�Ŀ�ȼ������������������ȫȼ�ա���������ͨ����������ʯ��ˮ�У��õ���ɫ��������Ϊ15g������������ʯ������ȼ�ղ������9.3g��
��1��ȼ�ղ�����ˮ������Ϊ_______ g��
��2����ԭ�����ǵ�һ���壬�������ʽΪ_____________________��
��3����ԭ�����������ֵ����ʵ�������̬����ɵĻ�����д�����ǵķ���ʽ__________________������д�����飩
��4����ԭ�����������ֵ����ʵ�����������ɵĻ�������ֻ��һ����������д�����ǵķ���ʽ____________________________������д�����飩
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2SO2(g) + O2(g) ![]() 2SO3(g)�ǹ�ҵ���������Ҫ��Ӧ֮һ��һ���¶��£��ڼס��ҡ��������ݻ���Ϊ2 L�ĺ����ܱ�������Ͷ��SO2(g)��O2(g)������ʼ���ʵ�����SO2��ƽ��ת�������±���ʾ�������ж��У���ȷ����
2SO3(g)�ǹ�ҵ���������Ҫ��Ӧ֮һ��һ���¶��£��ڼס��ҡ��������ݻ���Ϊ2 L�ĺ����ܱ�������Ͷ��SO2(g)��O2(g)������ʼ���ʵ�����SO2��ƽ��ת�������±���ʾ�������ж��У���ȷ����
�� | �� | �� | ||
��ʼ���ʵ��� | n(SO2) / mol | 0.4 | 0.8 | 0.8 |
n(O2) / mol | 0.24 | 0.24 | 0.48 | |
SO2��ƽ��ת���� / % | 80 | ��1 | ��2 | |
A. ���з�Ӧ��ƽ�ⳣ��С����
B. ƽ��ʱ������c(SO3)�Ǽ��е�2��
C. ���¶��£�ƽ�ⳣ��ֵΪ400
D. ƽ��ʱ������O2��ת���ʴ�������O2��ת����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijʵ��С������ͼ��ʾװ���Ʊ�һ�����ױ�(�����������ױ����������ױ�)��

��Ӧԭ����
ʵ���п����õ������ݣ�

���ԭ��������H-1 C-12 N-14
ʵ�鲽�裺��Ũ������Ũ���ᰴ�����1��3���ƻ����Һ�������ᣩ��40mL��
��������ƿ�м���13g�ױ����ӷ�������ͼ��ʾװ��ҩƷ������������
��������ƿ�м�����
�ܿ����¶�ԼΪ50�棬��Ӧ��Լ10 min������ƿ���д�������ɫ��״Һ����֣�
�ݷ����һ�����ױ������ᴿ���յõ�������һ�����ױ���15 g��
��ش��������⣺
(1)ʵ��ǰ��Ҫ������ƿ�м�������________��Ŀ����____________________��
(2)�����ܵ�������______________����ȴˮ�������ܵ�_______������a������b�����˽��롣
(3)����A��������________ ��ʹ�ø�����ǰ������еIJ�����_________________��
(4)���뷴Ӧ�����ķ������£�

���У�����1������Ϊ________������2����IJ��������оƾ��ơ��¶ȼơ���ƿ��ţ�ǹܣ�β�ӹܣ���________________��_________________��
(5)��ʵ���мױ���ת����Ϊ________���������3λ��Ч���֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�ϳ����ú����ˮ���� Na2S2O3��5H2O��ʵ���ҿ�����ͼװ��(��ȥ���ּг�����)ģ����������
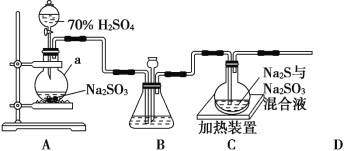
(1)����a��������__________��A�з����Ļ�ѧ��Ӧ����Ϊ_______________________��
(2)ʵ���У�Ϊʹ SO2 ��������װ��C�����õIJ���______________��װ��C���Ʊ���Ӧ����������___________________��
(3)װ�� C �е���Һһ��������ڼ��Ի����������Ʒ���ƣ������ӷ���ʽ��ʾ��ԭ��Ϊ ___________________��
(4)Ϊ����װ��C�п��ܳ��ֵ�Na2SO4���ʣ��ڲ��ı�ԭ��װ�õĻ�����Ӧ��ȡ�IJ�����__________________��
(5)װ�� D ���ڴ���β������ѡ�õ������װ��(�г���������ȥ)Ϊ_____(�����)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ء�����п����ػ�������;�dz��㷺���ش�����������
��1����̬пԭ�ӵļ۵����Ų�ʽΪ___________��K��F��Zn�ĵ縺�ԴӴ�С��˳��Ϊ___________��
��2��Zn��Caλ��ͬһ�������������������,�Ƶ��۵���е����п��,��ԭ����_______________��
��3��OF2���ӵļ��ι���Ϊ___________,����ԭ�ӵ��ӻ�����Ϊ___________��
��4��KOH ��O3��Ӧ�ɵõ�KO3(��������),KO3 �г�������,������___________;��O3-��Ϊ�ȵ�����ķ���Ϊ___________ (�� дһ�� )��
��5��K��F��Zn��ɵ�һ�־���ṹ��ͼ��ʾ,�侧������Ϊa=0.4058 nm��

��������Zn2+����λ��Ϊ___________����
�������н��ڵ�����F-��ľ���Ϊ_______________________(�г���ʽ����)nm��
���þ�����ܶ�Ϊ___________(�г���ʽ����,��NA��ʾ�����ӵ���������ֵ)g��cm-3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ݵ��ܱ������н��������������淴Ӧ��
����C(s)��H2O(g)![]() CO(g)��H2(g)
CO(g)��H2(g)
����CO(g)��H2O(g)![]() CO2(g)��H2(g)
CO2(g)��H2(g)
��������״̬���ٻ������ƽ����Է����������ٸı�
�ں���ʱ������ѹǿ���ٸı䡡�۸��������Ũ�����
�ܷ�Ӧ��ϵ���¶ȱ��ֲ��䡡�ݶ��������������Ƕ�����������ʵ�2������������ܶȲ��䡡�ߵ�λʱ���ڣ�����ˮ������������������������Ϊ9��1
�����ܱ����ס��������з�Ӧ���ﵽƽ��״̬����(����)
A. �٢ڢ� B. �ۢܢ� C. �ޢ� D. �ܢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com