
°ĺŐ‚ńŅ°Ņő“ĻķŅ∆—ßľ“ĹŤ÷ķ◊‘÷ų—–÷∆Ķń–¬–ÕőŔÓ‹ŐķļŌĹūīŖĽĮľŃĻ•ŅňŃňŅ…ŅōĹŠĻĻĶ•ĪŕŐľń…√◊Ļ‹Ķń÷∆Īłń—Ő‚°£ļ£Ķ◊Ĺū Ű»Ūńŗ «‘ŕļ£—ůĶ◊ł≤ł«◊ŇĶń“Ľ≤„ļž◊ō…ę≥ŃĽżőÔ£¨‘Ő≤ō◊ŇīůŃŅĶńĹū Ű◊ ‘ī£¨ļ¨”–őŔ°ĘŐķ°Ę√Ő°Ę–Ņ°ĘÓ‹Ķ»°£
£®1£©ĽýŐ¨łű‘≠◊”ĶńļňÕ‚őī≥…∂‘ĶÁ◊” żő™________°£Ķ•ĪŕŐľń…√◊Ļ‹Ņ…Ņī◊ų ĮńęŌ©—ō“Ľ∂®∑ĹŌÚĺŪ«ķ∂Ý≥…ĶńŅ’–ń‘≤÷ýŐŚ£¨∆šŐľ‘≠◊”Ķń‘”ĽĮ∑Ĺ Ĺő™________°£
£®2£©ń…√◊ĹŠĻĻ—űĽĮÓ‹Ņ…‘ŕ “ő¬Ō¬Ĺęľ◊ŃÚ»©![]() ÕÍ»ęīŖĽĮ—űĽĮ£¨ľ◊ŃÚ»©∑÷◊”Ķń÷––ń‘≠◊”ĶńVSEPRĻĻ–Õő™________£¨∆š∑÷◊”÷–
ÕÍ»ęīŖĽĮ—űĽĮ£¨ľ◊ŃÚ»©∑÷◊”Ķń÷––ń‘≠◊”ĶńVSEPRĻĻ–Õő™________£¨∆š∑÷◊”÷–![]() ľŁ”Ž
ľŁ”Ž![]() ľŁĶńłŲ żĪ»ő™________°£
ľŁĶńłŲ żĪ»ő™________°£
£®3£©ŃýŰ ĽýőŔ![]() Ķń»ŘĶ„ő™
Ķń»ŘĶ„ő™![]() £¨ «“Ľ÷÷÷ō“™ĶńőřĽķĹū ŰŇšļŌőÔ£¨Ņ…»‹”ŕ∂ŗ ż”–Ľķ»‹ľŃ°£»ż÷÷◊ť≥…‘™ňōĶńĶŕ“ĽĶÁņŽń‹”…–°ĶĹīůĶńň≥–Úő™________
£¨ «“Ľ÷÷÷ō“™ĶńőřĽķĹū ŰŇšļŌőÔ£¨Ņ…»‹”ŕ∂ŗ ż”–Ľķ»‹ľŃ°£»ż÷÷◊ť≥…‘™ňōĶńĶŕ“ĽĶÁņŽń‹”…–°ĶĹīůĶńň≥–Úő™________![]() ŐÓ‘™ňō∑ŻļŇ
ŐÓ‘™ňō∑ŻļŇ![]() °£ŇšŐŚCO÷–”ŽW–ő≥…ŇšőĽľŁĶń‘≠◊” «C∑«O£¨‘≠“Ú «________________________°£
°£ŇšŐŚCO÷–”ŽW–ő≥…ŇšőĽľŁĶń‘≠◊” «C∑«O£¨‘≠“Ú «________________________°£
£®4£©∂ŗ‘≠◊”∑÷◊”÷–łų‘≠◊”»Ű‘ŕÕ¨“Ľ∆Ĺ√śńŕ£¨«“”–ŌŗĽ•∆Ĺ––ĶńpĻžĶņ£¨‘ÚpĶÁ◊”Ņ…‘ŕ∂ŗłŲ‘≠◊”ľš‘ň∂Į£¨–ő≥…°įņŽ”Ú![]() ľŁ°Ī°£Ō¬Ń–∑÷◊”÷–īś‘ŕ°įņŽ”Ú
ľŁ°Ī°£Ō¬Ń–∑÷◊”÷–īś‘ŕ°įņŽ”Ú![]() ľŁ°ĪĶń”–________
ľŁ°ĪĶń”–________![]() ŐÓ◊÷ńł
ŐÓ◊÷ńł![]() °£
°£
A.Ľ∑ľļÕť ![]() ∂Ģ—űĽĮŃÚ
∂Ģ—űĽĮŃÚ ![]() »ż∑ķĽĮĶ™
»ż∑ķĽĮĶ™ ![]() ĪĹ∑”
ĪĹ∑”
£®5£©![]() °Ę
°Ę![]() ń‹”Ž
ń‹”Ž![]() ¬ÁļŌ–ő≥…¬ÁņŽ◊”£¨∆šĹŠĻĻ»ÁÕľ1ňý ĺ°£ł√¬ÁņŽ◊””ŽľōņŽ◊”Ņ…–ő≥…Ľ™ņ∂ľō—ő£¨ł√ľō—őĶńĽĮ—ß Ĺő™________°£
¬ÁļŌ–ő≥…¬ÁņŽ◊”£¨∆šĹŠĻĻ»ÁÕľ1ňý ĺ°£ł√¬ÁņŽ◊””ŽľōņŽ◊”Ņ…–ő≥…Ľ™ņ∂ľō—ő£¨ł√ľō—őĶńĽĮ—ß Ĺő™________°£
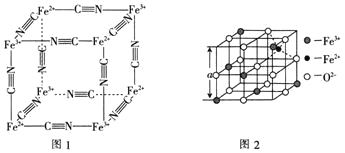
£®6£©Õľ2 «ī”Őķ—űŐŚņŽ◊”ĺßŐŚ![]() ÷–»°≥ŲĶńń‹ŐŚŌ÷∆šĺßŐŚĹŠĻĻĶń“ĽłŲŃĘ∑ĹŐŚ°£“—÷™
÷–»°≥ŲĶńń‹ŐŚŌ÷∆šĺßŐŚĹŠĻĻĶń“ĽłŲŃĘ∑ĹŐŚ°£“—÷™![]() ĺßŐŚĶń√‹∂»ő™
ĺßŐŚĶń√‹∂»ő™![]() £¨‘ÚÕľ2÷–
£¨‘ÚÕľ2÷–![]() ________
________![]() “—÷™
“—÷™![]() °£
°£
°ĺīūįł°Ņ![]()
![]() ∆Ĺ√ś»żĹ«–ő 1£ļ3
∆Ĺ√ś»żĹ«–ő 1£ļ3 ![]() C‘≠◊”įŽĺ∂Ī»Oīů£¨ĶÁłļ–‘ĹŌ–°£¨∂‘Ļ¬∂‘ĶÁ◊”ĶńőŁ“żŃ¶ĹŌ»ű£¨łŁ»›“◊–ő≥…ŇšőĽľŁ
C‘≠◊”įŽĺ∂Ī»Oīů£¨ĶÁłļ–‘ĹŌ–°£¨∂‘Ļ¬∂‘ĶÁ◊”ĶńőŁ“żŃ¶ĹŌ»ű£¨łŁ»›“◊–ő≥…ŇšőĽľŁ ![]()
![]()
![]()
°ĺĹ‚őŲ°Ņ
£®1£©Ő‚ł…łśňŖő“√«Ķ•ĪŕŐľń…√◊Ļ‹ «”√ ĮńęŌ©ĺŪ«ķ–ő≥…Ķń£¨ ĮńęŌ©ľīĶ•≤„Ķń Įńę£¨ Įńę√Ņ“Ľ≤„ « ∆Ĺ√śĹŠĻĻ£¨“ÚīňŌ‘∂Ý“◊ľŻ «
∆Ĺ√śĹŠĻĻ£¨“ÚīňŌ‘∂Ý“◊ľŻ «![]() ‘”ĽĮ£Ľ
‘”ĽĮ£Ľ
£®2£©—űļÕŃÚ «Õ¨◊Ś‘™ňō–‘÷ ŌŗĹŁ£¨“Úīňľ◊ŃÚ»©ÕÍ»ęŅ…“‘įī’’ľ◊»©ņī∑÷őŲ£Ľ
£®3£©Ķŕ“ĽĶÁņŽń‹Ņ…“‘įī’’ ßĶÁ◊”ń‹Ń¶£®ĽĻ‘≠–‘£©Ķń«Ņ»űņīŇ–∂Ō£¨ľīĶŕ“ĽĶÁņŽń‹‘ĹĶÕ‘Ĺ»›“◊ ßĶÁ◊”£Ľ»ŰŇšŐŚ‘≠◊”÷–”–∂ŗłŲ‘≠◊”ĺýń‹ŐŠĻ©Ļ¬∂‘ĶÁ◊”£¨‘Úň≠∂‘Ļ¬∂‘ĶÁ◊”ĶńőŁ“żŃ¶łŁ»ű£¨ň≠◊ŲŇšŐŚ‘≠◊”£Ľ
£®4£©łýĺ›∑÷◊”ĶńĹŠĻĻņī∑÷őŲ∆šīś≤Ľīś‘ŕŌŗĽ•∆Ĺ––ĶńpĻžĶņ£¨ľīŅ…Ň–∂Ōīś≤Ľīś‘ŕ°įņŽ”Ú![]() ľŁ°Ī£Ľ
ľŁ°Ī£Ľ
£®5£©∂•Ķ„…ŌĶń‘≠◊”įī![]() ņīň„£¨√ŅŐűĪŖ÷–ľšĶń‘≠◊”įī
ņīň„£¨√ŅŐűĪŖ÷–ľšĶń‘≠◊”įī![]() ņīň„ľīŅ…£¨◊Ę“‚«Ý∑÷
ņīň„ľīŅ…£¨◊Ę“‚«Ý∑÷![]() ļÕ
ļÕ![]() °£
°£
£®6£©Ő‚ńŅ÷–√‹∂»“—÷™£¨“Úīň÷Ľ“™įī’’![]() ņīĻĻ‘žĪŪīÔ ĹľīŅ…£¨◊Ę“‚Ķ•őĽĶńĽĽň„°£
ņīĻĻ‘žĪŪīÔ ĹľīŅ…£¨◊Ę“‚Ķ•őĽĶńĽĽň„°£
![]() łýĺ›ļťŐōĻś‘Ú£¨łű‘≠◊”ĶńľŘĶÁ◊”ŇŇ≤ľ Ĺő™
łýĺ›ļťŐōĻś‘Ú£¨łű‘≠◊”ĶńľŘĶÁ◊”ŇŇ≤ľ Ĺő™![]() £¨ĺý√Ľ”––ő≥…ĶÁ◊”∂‘£¨ňý“‘őī≥…∂‘ĶÁ◊” żő™6£Ľ ĮńęŌ© «∆Ĺ√śĹŠĻĻ£¨ňý“‘ł√Őľ‘≠◊”Ķń‘”ĽĮ∑Ĺ Ĺő™
£¨ĺý√Ľ”––ő≥…ĶÁ◊”∂‘£¨ňý“‘őī≥…∂‘ĶÁ◊” żő™6£Ľ ĮńęŌ© «∆Ĺ√śĹŠĻĻ£¨ňý“‘ł√Őľ‘≠◊”Ķń‘”ĽĮ∑Ĺ Ĺő™![]() £Ľ
£Ľ
![]() “ņ图Ř≤„ĶÁ◊”∂‘Ľ•≥‚ņŪ¬ŘĶ√÷™
“ņ图Ř≤„ĶÁ◊”∂‘Ľ•≥‚ņŪ¬ŘĶ√÷™![]() ∑÷◊”÷––ń‘≠◊” «
∑÷◊”÷––ń‘≠◊” «![]() ‘”ĽĮ£¨÷––ń‘≠◊”ĶńVSEPRĻĻ–Õő™∆Ĺ√ś»żĹ«–ő£¨∆š∑÷◊”÷–
‘”ĽĮ£¨÷––ń‘≠◊”ĶńVSEPRĻĻ–Õő™∆Ĺ√ś»żĹ«–ő£¨∆š∑÷◊”÷–![]() ľŁ”Ž
ľŁ”Ž![]() ľŁĶń żńŅ∑÷Īū «1ļÕ3£Ľ
ľŁĶń żńŅ∑÷Īū «1ļÕ3£Ľ
![]() ◊ÓÕ‚≤„ĶÁ◊” żő™2£¨ĹŌC°ĘOŃĹ‘≠◊”ĶńĶŕ“ĽĶÁņŽń‹“™–°£¨C°ĘOŃĹ‘≠◊”ĶńļňÕ‚ĶÁ◊”ŇŇ≤ľ Ĺ∑÷Īūő™
◊ÓÕ‚≤„ĶÁ◊” żő™2£¨ĹŌC°ĘOŃĹ‘≠◊”ĶńĶŕ“ĽĶÁņŽń‹“™–°£¨C°ĘOŃĹ‘≠◊”ĶńļňÕ‚ĶÁ◊”ŇŇ≤ľ Ĺ∑÷Īūő™![]() °Ę
°Ę![]() £¨ňý“‘O‘≠◊”ĶńĶŕ“ĽĶÁņŽń‹łŁīů£Ľ‘ŕŃýŰ ĽýőŔ
£¨ňý“‘O‘≠◊”ĶńĶŕ“ĽĶÁņŽń‹łŁīů£Ľ‘ŕŃýŰ ĽýőŔ![]() ∑÷◊”÷–£¨W «÷––ń‘≠◊”£¨CO «ŇšŐŚ£¨”…”ŕC‘≠◊”įŽĺ∂Ī»Oīů£¨ĶÁłļ–‘–°£¨∂‘Ļ¬∂‘ĶÁ◊”ĶńőŁ“żŃ¶ĹŌ»ű£¨łŁ»›“◊–ő≥…ŇšőĽľŁ£¨ňý“‘ŇšŐŚCO÷–”ŽW–ő≥…ŇšőĽĶń‘≠◊” «C∑«O£Ľ
∑÷◊”÷–£¨W «÷––ń‘≠◊”£¨CO «ŇšŐŚ£¨”…”ŕC‘≠◊”įŽĺ∂Ī»Oīů£¨ĶÁłļ–‘–°£¨∂‘Ļ¬∂‘ĶÁ◊”ĶńőŁ“żŃ¶ĹŌ»ű£¨łŁ»›“◊–ő≥…ŇšőĽľŁ£¨ňý“‘ŇšŐŚCO÷–”ŽW–ő≥…ŇšőĽĶń‘≠◊” «C∑«O£Ľ
![]() ∂ŗ‘≠◊”∑÷◊”÷–łų‘≠◊”»Ű‘ŕÕ¨“Ľ∆Ĺ√śńŕ£¨«“”–ŌŗĽ•∆Ĺ––ĶńpĻžĶņ£¨‘ÚpĶÁ◊”Ņ…‘ŕ∂ŗłŲ‘≠◊”ľš‘ň∂Į£¨–ő≥…°įņŽ”Ú
∂ŗ‘≠◊”∑÷◊”÷–łų‘≠◊”»Ű‘ŕÕ¨“Ľ∆Ĺ√śńŕ£¨«“”–ŌŗĽ•∆Ĺ––ĶńpĻžĶņ£¨‘ÚpĶÁ◊”Ņ…‘ŕ∂ŗłŲ‘≠◊”ľš‘ň∂Į£¨–ő≥…°įņŽ”Ú![]() ľŁ°Ī£¨
ľŁ°Ī£¨
A.Ľ∑ľļÕť∑÷◊”÷–÷Ľīś‘ŕ![]() ľŁ£¨≤Ľīś‘ŕ
ľŁ£¨≤Ľīś‘ŕ![]() ľŁ£¨Ļ AīŪőů£Ľ
ľŁ£¨Ļ AīŪőů£Ľ
B.∂Ģ—űĽĮŃÚ∑÷◊”÷–Ķń÷––ń‘≠◊”S”ŽŃĹłŲO‘≠◊”÷–”–ŌŗĽ•∆Ĺ––ĶńpĻžĶņ£¨Ņ…“‘–ő≥…°įņŽ”Ú![]() ľŁ°Ī£¨∆š°įņŽ”Ú
ľŁ°Ī£¨∆š°įņŽ”Ú![]() ľŁ°Īő™
ľŁ°Īő™![]() £¨Ļ B’ż»∑£Ľ
£¨Ļ B’ż»∑£Ľ
C.»ż∑ķĽĮĶ™∑÷◊”÷–÷Ľīś‘ŕ![]() ľŁ£¨≤Ľīś‘ŕ
ľŁ£¨≤Ľīś‘ŕ![]() ľŁ£¨Ļ CīŪőů£Ľ
ľŁ£¨Ļ CīŪőů£Ľ
D.ĪĹ∑”∑÷◊”÷–ĶńĪĹĽ∑…ŌĶńC‘≠◊””ŽŰ«ĽýO‘≠◊”÷–”–ŌŗĽ•∆Ĺ––ĶńpĻžĶņ£¨Ņ…“‘–ő≥…°įņŽ”Ú![]() ľŁ°Ī£¨∆š°įņŽ”Ú
ľŁ°Ī£¨∆š°įņŽ”Ú![]() ľŁ°Īő™
ľŁ°Īő™![]() £¨Ļ D’ż»∑£¨
£¨Ļ D’ż»∑£¨
īūįł—°BD£Ľ
![]() łýĺ›Õľ1ņŽ◊”ĺßįŻĹŠĻĻ∑÷őŲ£¨
łýĺ›Õľ1ņŽ◊”ĺßįŻĹŠĻĻ∑÷őŲ£¨![]() °Ę
°Ę![]() ń‹”Ž
ń‹”Ž![]() ¬ÁļŌļů–ő≥…ĶńņŽ◊”ő™
¬ÁļŌļů–ő≥…ĶńņŽ◊”ő™![]() £¨ňý“‘ł√ņŽ◊”–ő≥…Ķńľō—őĶńĽĮ—ß Ĺő™
£¨ňý“‘ł√ņŽ◊”–ő≥…Ķńľō—őĶńĽĮ—ß Ĺő™![]() £¨ľī
£¨ľī![]() £¨ĽÚŅ…–īő™
£¨ĽÚŅ…–īő™![]() £Ľ
£Ľ
![]() Õľ2÷–
Õľ2÷–![]() Ķń żńŅő™
Ķń żńŅő™![]() £¨
£¨![]() őĽ”ŕŃĘ∑ĹŐŚńŕ£¨ żńŅő™1£¨—űņŽ◊”Ķń żńŅő™
őĽ”ŕŃĘ∑ĹŐŚńŕ£¨ żńŅő™1£¨—űņŽ◊”Ķń żńŅő™![]() £Ľłýĺ›√‹∂»
£Ľłýĺ›√‹∂»![]() Ņ…Ķ√
Ņ…Ķ√![]() £¨ľ∆ň„Ķ√
£¨ľ∆ň„Ķ√![]() °£
°£



| ńÍľ∂ | łŖ÷–Ņő≥Ő | ńÍľ∂ | ≥ű÷–Ņő≥Ő |
| łŖ“Ľ | łŖ“Ľ√‚∑—Ņő≥ŐÕ∆ľŲ£° | ≥ű“Ľ | ≥ű“Ľ√‚∑—Ņő≥ŐÕ∆ľŲ£° |
| łŖ∂Ģ | łŖ∂Ģ√‚∑—Ņő≥ŐÕ∆ľŲ£° | ≥ű∂Ģ | ≥ű∂Ģ√‚∑—Ņő≥ŐÕ∆ľŲ£° |
| łŖ»ż | łŖ»ż√‚∑—Ņő≥ŐÕ∆ľŲ£° | ≥ű»ż | ≥ű»ż√‚∑—Ņő≥ŐÕ∆ľŲ£° |
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°ŅĘŮ. (1) “—÷™S4ĶńĹŠĻĻ Ĺ»ÁÕľ£¨∑ī”¶S4(g) + 4Cl2(s )== 4SCl2(g) °ųH= - 4 kJ°§mol-1£¨S°™SľŁĶńľŁń‹ő™266 kJ°§mol-1£¨S°™ClľŁĶńľŁń‹ő™255 kJ°§mol-1£¨‘Ú1mol Cl2(g)∑÷◊”÷–ĶńĽĮ—ßľŁ∂ŌŃ— Ī–Ť“™őŁ ’Ķńń‹ŃŅő™_____kJ°£
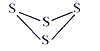
ĘÚ. Ļ§“Ķ…ŌļŌ≥…¬»ĽĮ—«ŪŅ∑ī”¶£ļSO2(g)+ SCl2(g)+Cl2(s)![]() 2SOCl2(g)£¨ł√∑ī”¶÷–ń≥“Ľ∑ī”¶őÔĶńŐŚĽż∑÷ ż(“‘A%ĪŪ ĺ)ňśő¬∂»ĶńĪšĽĮĻōŌĶ»ÁÕľňý ĺ°£
2SOCl2(g)£¨ł√∑ī”¶÷–ń≥“Ľ∑ī”¶őÔĶńŐŚĽż∑÷ ż(“‘A%ĪŪ ĺ)ňśő¬∂»ĶńĪšĽĮĻōŌĶ»ÁÕľňý ĺ°£
(2) ‘ŕ373K Ī£¨ŌÚ2L√‹Ī’»›∆ų÷–Õ®»ŽőÔ÷ ĶńŃŅĺýő™0.04 molĶńSO2°ĘSCl2”ŽCl2£¨ ∑Ę…ķ…Ō Ų∑ī”¶°£≤‚Ķ√∆š—Ļ«Ņ(p)ňś Īľš(t)ĶńĪšĽĮő™Ō¬ĪŪ÷– żĺ›ĘŮ(∆Ĺļ‚ ĪĶńő¬∂»”Ž∆ū ľő¬∂»ŌŗÕ¨)
t/min | 0 | 1 | 2 | 3 | 4 | 5 |
ĘŮ | 6.0p0 | 6.7 p0 | 6.1 p0 | 5.4 p0 | 5.0 p0 | 5.0 p0 |
ĘÚ | 6.0 p0 | 7.0 p0 | 5.3 p0 | 5.0 p0 | 5.0 p0 | 5.0 p0 |
ĘŔł√∑ī”¶Ķń°ųH_____0(ŐÓ°į£ĺ°Ī°į£ľ°ĪĽÚ°į=°Ī)°£
Ęŕ∑ī”¶Ņ™ ľ÷ŃīÔĶĹ∆Ĺļ‚ Ī£¨v(SCl2)=__________°£
ĘŘ»Ű÷ĽłńĪšń≥“ĽŐűľĢ£¨≤‚Ķ√∆š—Ļ«Ņňś ĪľšĶńĪšĽĮő™ĪŪ÷– żĺ›ĘÚ£¨‘ÚłńĪšĶńŐűľĢ «_______________°£
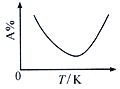
(3) Ō¬Õľ «ń≥Õ¨—ß≤‚∂®Ķń…Ō Ų∑ī”¶ĶńpK(pK= - lgK)”Žő¬∂»ĶńĪšĽĮĻōŌĶÕľ°£

ĘŔ AĶ„Ķń ż÷Ķő™_________(“—÷™lg4=0.6)°£
ĘŕĶĪ…żłŖĶĹń≥“Ľő¬∂» Ī£¨∑ī”¶÷ō–¬īÔĶĹ∆Ĺļ‚£¨AĶ„Ņ…ń‹ĪšĽĮő™______Ķ„°£
III. (4) ĶÁĹ‚NO2÷∆ĪłNH4NO3£¨∆šĻ§◊ų‘≠ņŪ»ÁÕľňý ĺ°£
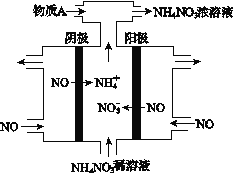
ĘŔ“űľęĶńĶÁľę∑ī”¶ Ĺő™_________________________°£
Ęŕő™ ĻĶÁĹ‚≤ķőÔ»ę≤Ņ◊™ĽĮő™NH4NO3£¨–Ť≤Ļ≥šń≥÷÷őÔ÷ A£¨‘ÚAĶńĽĮ—ß Ĺő™___________°£
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°Ņ≥£ő¬Ō¬£¨ŌÚ![]() Ķń
Ķń![]() »‹“ļ÷–÷ūĶőĶőľ”
»‹“ļ÷–÷ūĶőĶőľ”![]() Ķń
Ķń![]() »‹“ļ°£»‹“ļ
»‹“ļ°£»‹“ļ![]() ňśĶő»Ž
ňśĶő»Ž![]() »‹“ļŐŚĽżĪšĽĮ»ÁÕľňý ĺ°£Ō¬Ń–ňĶ∑®’ż»∑Ķń «£® £©
»‹“ļŐŚĽżĪšĽĮ»ÁÕľňý ĺ°£Ō¬Ń–ňĶ∑®’ż»∑Ķń «£® £©
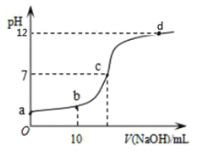
A.![]() Ķ„Ķń
Ķ„Ķń![]()
B.![]() Ķ„ Ī£¨
Ķ„ Ī£¨![]()
C.![]() Ķ„ Ī£¨
Ķ„ Ī£¨![]()
D.ī”a°ķd£¨ňģĶńĶÁņŽ∂»Ō»‘Ųīůļůľű–°
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°ŅĹęĶ»őÔ÷ ĶńŃŅĶńA°ĘBŃĹ÷÷∆ÝŐŚĽžļŌ”ŕ2LĶń√‹Ī’»›∆ų÷–£¨∑Ę…ķ»ÁŌ¬∑ī”¶£ļ3A(g) + B(g) ![]() x C(g) + 2D(g)°£ĺ≠4minīÔĶĹĽĮ—ß∆Ĺļ‚°£īň Ī≤‚Ķ√DĶńŇ®∂»ő™0.5mol/L£¨«“c(A)£ļc(B) = 3£ļ5£¨CĶń∆ĹĺýňŔ¬ ő™0.125mol/L£¨‘Ú£¨
x C(g) + 2D(g)°£ĺ≠4minīÔĶĹĽĮ—ß∆Ĺļ‚°£īň Ī≤‚Ķ√DĶńŇ®∂»ő™0.5mol/L£¨«“c(A)£ļc(B) = 3£ļ5£¨CĶń∆ĹĺýňŔ¬ ő™0.125mol/L£¨‘Ú£¨
£®1£©īň ĪAĶńőÔ÷ ĶńŃŅŇ®∂»ő™_______________°£
£®2£©BĶń∆ĹĺýňŔ¬ ő™_______________°£
£®3£©xĶń÷Ķő™_________________°£
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°ŅŌ¬Ń––ū Ų÷–£¨īŪőůĶń «£® £©
A.ń¶∂Ż «őÔ÷ ĶńŃŅĶńĶ•őĽ
B.36gňģ÷–ļ¨«‚‘≠◊” żńŅő™4NA£®NAĪŪ ĺįĘ∑Łľ”Ķ¬¬ř≥£ żĶń÷Ķ£©
C.‘ŕ0.5molNa2SO4÷–£¨ļ¨”–ĶńNa+ ż‘ľ «6.02°Ń1023
D.Ķ»÷ ŃŅĶńO2”ŽO3£¨ňýļ¨—ű‘≠◊” ż÷ģĪ»ő™3£ļ2
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°Ņ“‘ŐĢAő™‘≠ŃŌļŌ≥…“Ĺ”√¬ť◊Ū“©‹–◊ŰŅ®“ÚļÕ ≥∆∑∑ņłĮľŃńŠ≤īĹūňŠ““ű•Ķń¬∑ŌŖ»ÁŌ¬£ļ
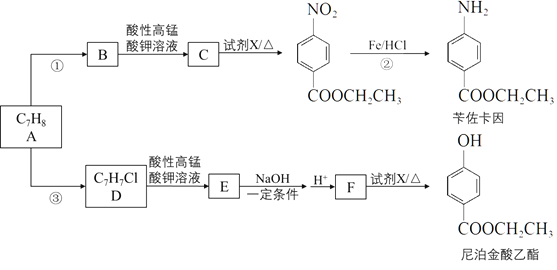
ÕÍ≥…Ō¬Ń–ŐÓŅ’
£®1£©AĶńĹŠĻĻľÚ Ĺ «____________°£Ļ§“Ķ…ŌAĶńņī‘īÕ®≥£ «_______________°£
£®2£©∑ī”¶ĘŔĶńĽĮ—ß∑Ĺ≥Ő Ĺ «_____________________°£
£®3£©∑ī”¶ĘŕĶń∑ī”¶ņŗ–Õ «____________°£
£®4£©∑ī”¶ĘŘĶń ‘ľŃ”ŽŐűľĢ «_______________°£
£®5£©FĶńĹŠĻĻľÚ Ĺ «_____________________°£
£®6£©ńŠ≤īĹūňŠ““ű•”–∂ŗ÷÷Õ¨∑÷“žĻĻŐŚ£¨»ő–ī“Ľ÷÷∑ŻļŌŌ¬Ń–“™«ůĶńÕ¨∑÷“žĻĻŐŚĶńĹŠĻĻľÚ Ĺ____________°£
i. ń‹∑Ę…ķ“ÝĺĶ∑ī”¶«“∑÷◊”÷–≤Ľļ¨ľ◊Ľý
ii. ĪĹĽ∑…Ō”–ŃĹłŲ∂‘őĽ»°īķĽý
£®7£©“‘Aő™∆ū ľ‘≠ŃŌ£¨Ņ…“‘ļŌ≥…ÕŅłń“ļĶń÷ų“™≥…∑÷—«ľ◊ĽýĽ∑ľļÕť£®![]() £©£¨–ī≥Ų∆šļŌ≥…ŌŖ¬∑°£___________________°£
£©£¨–ī≥Ų∆šļŌ≥…ŌŖ¬∑°£___________________°£
£®ļŌ≥…¬∑ŌŖ≥£”√ĶńĪŪ ĺ∑Ĺ Ĺő™£ļ![]() £©
£©
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°ŅA «ĽĮ—ß Ķ—ť “÷–◊Ó≥£ľŻĶń”–ĽķőÔ£¨ňŁ“◊»‹”ŕňģ≤Ę”–Őō ‚Ō„ő∂£¨≤Ęń‹ĹÝ––»ÁÕľňý ĺĶń∂ŗ÷÷∑ī”¶°£
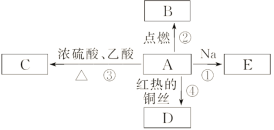
(1)–ī≥ŲAĶńĹŠĻĻľÚ Ĺ£ļ______________________£¨∆šĻŔń‹ÕŇ√Ż≥∆ő™__________°£
(2)Ō¬Ń–∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺ£ļ
∑ī”¶ĘŔ£ļ____________________________________________£Ľ
∑ī”¶ĘŘ£ļ___________________________________________£Ľ
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°ŅőŪŲ≤Őž∆Ý—Ō÷ō”įŌž»ň√«Ķń…ķĽÓļÕĹ°ŅĶ°£∆š÷– ◊“™őŘ»ĺőÔő™Ņ…őŁ»ŽŅŇŃ£őÔPM2.5£¨∆š÷ų“™ņī‘īő™»ľ√ļ°ĘĽķ∂Į≥Ķő≤∆ÝĶ»°£“Úīňłń…∆ń‹‘īĹŠĻĻ°ĘĽķ∂Į≥ĶŌřļŇĶ»īŽ ©ń‹”––ßľű…ŔPM2.5°ĘSO2°ĘNOxĶ»őŘ»ĺ°£
«ŽĽōīūŌ¬Ń–ő Ő‚£ļ
£®1£©∆Ż≥Ķő≤∆Ý÷–NOxļÕCOĶń…ķ≥…£ļ“—÷™∆Żł◊÷–…ķ≥…NOĶń∑ī”¶ő™£ļN2(g)£ęO2(g)![]() 2NO(g)°™Q°£ļ„ő¬°Ęļ„»›√‹Ī’»›∆ų÷–£¨Ō¬Ń–ňĶ∑®÷–£¨ń‹ňĶ√ųł√∑ī”¶īÔĶĹĽĮ—ß∆Ĺļ‚◊īŐ¨Ķń «____°£
2NO(g)°™Q°£ļ„ő¬°Ęļ„»›√‹Ī’»›∆ų÷–£¨Ō¬Ń–ňĶ∑®÷–£¨ń‹ňĶ√ųł√∑ī”¶īÔĶĹĽĮ—ß∆Ĺļ‚◊īŐ¨Ķń «____°£
A.ĽžļŌ∆ÝŐŚĶń√‹∂»≤Ľ‘ŔĪšĽĮ
B.ĽžļŌ∆ÝŐŚĶń—Ļ«Ņ≤Ľ‘ŔĪšĽĮ
C.N2°ĘO2°ĘNOĶńőÔ÷ ĶńŃŅ÷ģĪ»ő™1°√1°√2
D.—ű∆ÝĶń◊™ĽĮ¬ ≤Ľ‘ŔĪšĽĮ
£®2£©∆Ż≥Ķ Ļ”√““īľ∆Ż”Õ≤Ę≤Ľń‹ľű…ŔNOxĶńŇŇ∑Ň£¨’‚ ĻNOxĶń”––ßŌŻ≥ż≥…ő™Ľ∑Ī£Ńž”ÚĶń÷ō“™ŅőŐ‚°£ń≥—–ĺŅ–‘–°◊ť‘ŕ Ķ—ť ““‘Ag°™ZSM°™5ő™īŖĽĮľŃ£¨≤‚Ķ√NO◊™ĽĮő™N2Ķń◊™ĽĮ¬ ňśő¬∂»ĪšĽĮ«ťŅŲ»ÁÕľňý ĺ°£»Ű≤Ľ Ļ”√CO£¨ő¬∂»≥¨Ļż775 K£¨∑ĘŌ÷NOĶń∑÷Ĺ‚¬ ĹĶĶÕ£¨∆šŅ…ń‹Ķń‘≠“Úő™___£¨‘ŕ![]() £Ĺ1ĶńŐűľĢŌ¬£¨ő™łŁļ√Ķń≥ż»•NOx£¨”¶Ņō÷∆Ķń◊Óľ—ő¬∂»‘ŕ___K◊ů”“°£
£Ĺ1ĶńŐűľĢŌ¬£¨ő™łŁļ√Ķń≥ż»•NOx£¨”¶Ņō÷∆Ķń◊Óľ—ő¬∂»‘ŕ___K◊ů”“°£

£®3£©≥ĶŃĺŇŇ∑ŇĶńĶ™—űĽĮőÔ°Ę√ļ»ľ…’≤ķ…ķĶń∂Ģ—űĽĮŃÚ «Ķľ÷¬őŪŲ≤Őž∆ÝĶń°į◊ÔŅżĽŲ ◊°Ī°£ĽÓ–‘ŐŅŅ…ī¶ņŪīů∆ÝőŘ»ĺőÔNO°£‘ŕ5L√‹Ī’»›∆ų÷–ľ”»ŽNOļÕĽÓ–‘ŐŅ(ľŔ…Ťőř‘”÷ )£¨“Ľ∂®ŐűľĢŌ¬…ķ≥…∆ÝŐŚEļÕF°£ĶĪő¬∂»∑÷Īū‘ŕT1°śļÕT2°ś Ī£¨≤‚Ķ√łųőÔ÷ ∆Ĺļ‚ ĪőÔ÷ ĶńŃŅ(n/mol)»ÁŌ¬ĪŪ£ļ
őÔ÷ ő¬∂»(°ś) | ĽÓ–‘ŐŅ | NO | E | F |
≥ű ľ | 3.000 | 0.10 | 0 | 0 |
T1 | 2.960 | 0.020 | 0.040 | 0.040 |
T2 | 2.975 | 0.050 | 0.025 | 0.025 |
ĘŔ–ī≥ŲNO”ŽĽÓ–‘ŐŅ∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺ£ļ___°£
Ęŕ»ŰT1£ľT2£¨‘Úł√∑ī”¶ĶńQ__0(ŐÓ°į£ĺ°Ī°į£ľ°ĪĽÚ°į£Ĺ°Ī)°£
ĘŘ…Ō Ų∑ī”¶T1°ś ĪīÔĶĹĽĮ—ß∆Ĺļ‚ļů‘ŔÕ®»Ž0.1molNO∆ÝŐŚ£¨‘ÚīÔĶĹ–¬ĽĮ—ß∆Ĺļ‚ ĪNOĶń◊™ĽĮ¬ ő™___°£
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°ŅŌ¬Ń– ¬ Ķ≤Ľń‹”√ņ’ŌńŐōŃ–‘≠ņŪĹ‚ ÕĶń «£®£©
A.Ľ∆¬Ő…ęĶń¬»ňģĻ‚’’ļů—’…ęĪš«≥
B.ļž◊ō…ęĶń∂Ģ—űĽĮĶ™ľ”—Ļļů—’…ęŌ»Īš…Ó‘ŔĪš«≥
C.ļŌ≥…įĪĻ§“Ķ Ļ”√īŖĽĮľŃŐŠłŖįĪĶń≤ķŃŅ
D.Ļ§“Ķ…Ō…ķ≤ķŃÚňŠĶńĻż≥Ő÷–£¨ Ļ”√ĻżŃŅĶńŅ’∆Ý“‘ŐŠłŖ∂Ģ—űĽĮŃÚĶńņŻ”√¬
≤ťŅīīūįłļÕĹ‚őŲ>>
Ļķľ —ß–£”Ň—° - Ń∑Ōį≤ŠŃ–ĪŪ - ‘Ő‚Ń–ĪŪ
ļĢĪĪ °Ľ•Ń™ÕÝő•∑®ļÕ≤ĽŃľ–ŇŌĘĺŔĪ®∆ĹŐ® | ÕÝ…Ō”–ļ¶–ŇŌĘĺŔĪ®◊®«Ý | ĶÁ–Ň’©∆≠ĺŔĪ®◊®«Ý | …śņķ ∑–ťőř÷ų“Ś”–ļ¶–ŇŌĘĺŔĪ®◊®«Ý | …ś∆ů«÷»®ĺŔĪ®◊®«Ý
ő•∑®ļÕ≤ĽŃľ–ŇŌĘĺŔĪ®ĶÁĽį£ļ027-86699610 ĺŔĪ®” Ōš£ļ58377363@163.com