
��1�������������ʣ���NaCl���� ��SO2 ��ϡ���� ��ʯī ��BaSO4���� ������(C12H22O11) �߾ƾ� �����ڵ�KNO3 ��CaO �ⴿ���Ĵ���
��ش��������⣨����ţ���
�����������ܵ������ ���������������ڵ���ʵ��� ��
��2����Ҫ��д�����ж�Ӧ�ķ���ʽ��
���ٵ��뷽��ʽ���ڻ�ѧ����ʽ�������ӷ���ʽ��
��Al2(SO4)3��
��CO2+2OH-=CO32-+H2O��
��NaHCO3��NaHSO4��Һ��Ӧ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�弰�仯����㷺Ӧ�����л��ϳɡ���ѧ����������
��1����ˮ�����������Ԫ�صı仯���£�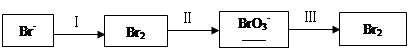
�ٹ��̢�ˮ�Լ��ԣ�����pH��3.5����ͨ��������
��.ͨ��������Ӧ�����ӷ���ʽ��______��
��.����ˮpH�����Cl2�������ʣ���ƽ��ԭ��������ԭ����______��
�ڹ��̢����ȿ�������ϳ�������Ũ̼������Һ���ա���ɲ���ƽ���з���ʽ��
Br2�� Na2CO3��
Na2CO3�� NaBrO3��
NaBrO3�� CO2��
CO2�� ______
______
�۹��̢��������ữ�ɵ�Br2��Na2SO4�Ļ����Һ��
��ͬ�����£����������ữ������������������٣�ԭ����______��
��2��NaBrO3��һ�ַ����Լ����������ữ��NaI��Һ����μ���NaBrO3��Һ��������2.6 mol NaBrO3ʱ����÷�Ӧ����Һ����͵�Ĵ�����ʽ�����ʵ����ֱ�Ϊ��
| ���� | I2 | Br2 | IO3- |
| ���ʵ���/mol | 0.5 | 1.3 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������������:
���� ������ ��CO2 ��H2SO4 ��Ba(OH)2 ���ɫ�������������� ��HCl
��1���������������ڵ���ʵ��� ��(�����)
��2�������Һ���μӢߵ���Һ,������������ ��
��3������������������������ˮ��Һ�з�����Ӧ,�����ӷ���ʽΪ:H++OH-=H2O,��÷�Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��װ�����Ӻ������������ձ��м���20mL���ҵ�Ba(OH)2��Һ����ͨ��Դ���μ�ϡ������������
��1�����μ�ϡ����ʱ���ձ��й۲쵽�������ǣ� ��
��2�����ݵ������ǣ� ��ԭ���ǣ� ��
��3��������Ӧ�����ӷ���ʽΪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������͵�������(NOx)�Դ�����Ⱦ�������أ��о�����������Ⱦ�ķ����ǻ�ѧ�����ߵ���Ҫ���⣬Ŀǰ�кܶ��ַ�������������Ⱦ��
��1�������ü������ԭNOx�ķ�������NOx����Ӧ���£�
CH4(g)+4NO2(g)�� 4NO(g)+CO2(g)+2H2O(g)����H�� ��574 kJ��mol��1
CH4(g)+4NO(g)�� 2N2(g)+CO2(g)+2H2O(g)����H�� ��1160 kJ��mol��1
��CH4(g)+2NO2(g)�� N2(g)+CO2(g)+2H2O(g)����H�� ��
��2����������β���ķ���֮һ�����������ϰ�װ��ת�������������·�Ӧ��2NO(g)+2CO(g) N2(g)+2CO2(g)����H��0��
N2(g)+2CO2(g)����H��0��
����һ���¶��£���2molNO��1molCO����1L�̶��ݻ��������У�15���Ӻ�ﵽƽ�⣬��Ӧ�����и����ʵ�Ũ�ȱ仯��ͼ����ʾ����ƽ�ⳣ��K= (С�������3λ)��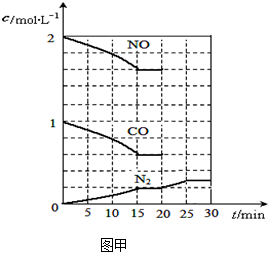
���������¶Ȳ��䣬20minʱ���������г���CO����0.6mol��ƽ�⽫ �ƶ�(����������ҡ�����)��
���������¶Ȳ��䣬20minʱ��ԭƽ�������г���CO��N2��0.6mol��ƽ�⽫ �ƶ�(����������ҡ�����)��
��20minʱ�����ı䷴Ӧ����������N2Ũ�ȷ�����ͼ��ʾ�ı仯����ı������������
(����ĸ)��
| A��������� | B�������¶� | C����С������� | D������CO2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��M��N������Һ�����ⶨ��������Һ�к�������12�����ӣ�Al3����Cl����Na����K����NO3-��OH����Fe2����AlO2-��CO32-��NH4+��SO42-��H����
(1)������б�����ʵ��ٵĽ��ۺ�ʵ��ڵ�ʵ�������Լ�����
| ʵ�������Լ����� | ���� |
| ��ȡ����N��Һ�μ����������ᱵ��Һ���������� | N�в��� ���� |
| �� | ȷ��M��Һ�к���Na��������K�� |
 ����pH��ֽ���M��Һ��pH��ֽ����ɫ ����pH��ֽ���M��Һ��pH��ֽ����ɫ | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ������������
(l)д���������ʵĵ��뷽��ʽ��
Fe2(SO4)3__________________________________________________��
NaHCO3______________________________________________________��
(2)д�����з�Ӧ�����ӷ���ʽ��
ϡ������̼��Ʒ�Ӧ____________________________________________��
����������Һ��ϡ���ᷴӦ_______________________________________��
(3)д�����������ӷ���ʽ���Ӧ�Ļ�ѧ����ʽ��
H����OH��=H2O __________________________________________��
CO32����2H��=CO2����H2O__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������з�����ij��ѧ��Ӧ����÷�Ӧ�����У������д���Na+��H2O��CN-��ClO-��HCO3-��N2��Cl-�������ӣ�����ClO-��N2�����ʵ�����ʱ��仯��������ͼ��ʾ��������������������: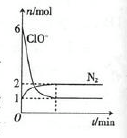
��1��CNһ�е�Ԫ�صĻ��ϼ��� ��
��2���������з�����Ӧ�����ӷ���ʽ�� ���÷�Ӧ������������ (�ѧʽ)��
(3)����������ClO2����ClO-����Һ�ʼ��ԣ���������������N2�����ʵ�����ͬ����ס����������вμӷ�Ӧ�������������ʵ���֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪ij��ɫ��Һ�п��ܺ���Na+��Ba2+��Fe3+��MnO4����SO32����Cl����HCO3�� �еļ��֣����ν�������ʵ�飬�۲쵽���������£�����PH��ֽ���飬��Һ��PH��7��
������Һ�еμ���ˮ��û������������õ���ɫ��Һ��
����ڵ���Һ�еμ������ữ��������Һ��������ɫ������
����ڵ���Һ�еμ�BaCl2��Һ����������������İ�ɫ������
��1��ԭ��Һ��һ�����е��������� ���϶�û�е��������� ��
��2������ڵ����ӷ�Ӧ����ʽΪ ��
��3���ܷ�ȷ������������ �������У�д�����ӷ��ţ�û����дû�У����������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com