
 �Ȼ������ε�ⷨ�����Ȼ���Ϊԭ�ϣ��Լ��������������Ȼ��������MgCl2��KCl��CaCl2��Ϊ����ʽ��е����ȡ���ķ�����
�Ȼ������ε�ⷨ�����Ȼ���Ϊԭ�ϣ��Լ��������������Ȼ��������MgCl2��KCl��CaCl2��Ϊ����ʽ��е����ȡ���ķ�����
| �� |
| �� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
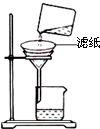
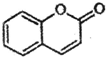 �� ��һ�ִ�����ijЩֲ��Ĺ��������е���Ȼ���ϣ���֪��
�� ��һ�ִ�����ijЩֲ��Ĺ��������е���Ȼ���ϣ���֪��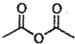 ��
��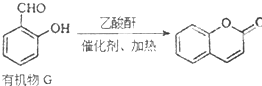
 ����һ�ֳ����л��ܼ����ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
����һ�ֳ����л��ܼ����ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�| HBr |
| NaOH��Һ |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ���� |
| �ȿ����� |
| ˮ���� |
| ��Һ |
| ���� |
| �ữ |
| ���� |
| ���� |
| ˮ |
| ��ˮ |
| ���� |
| ���� |
| �ۻ� |
| ��� |
| �ŵ� |
| ˮ |
| ���� |
| һ�� |
| ������ |
| �ϳ��� |
| �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ͼ1��ʾij�ֺ����л�������Ľṹ���������4����ԭ�ӷֱ�λ�����������4�����㣨��ͼ2���������ڴ��ڿ�ǻ����Ƕ��ij�����γ�4���������ʶ���������У��ܱ����л�������ʶ����ǣ�������
ͼ1��ʾij�ֺ����л�������Ľṹ���������4����ԭ�ӷֱ�λ�����������4�����㣨��ͼ2���������ڴ��ڿ�ǻ����Ƕ��ij�����γ�4���������ʶ���������У��ܱ����л�������ʶ����ǣ�������| A��CF4 |
| B��CH4 |
| C��NH4+ |
| D��H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | �����Ϣ |
| X | ����������Ӧ��ˮ���ﻯѧʽΪH2XO3 |
| Y | �����������Ǵ�����3�� |
| Z | ������Ԫ����ԭ�Ӱ뾶��������Ԫ�� |
| W | һ�ֺ��ص�������Ϊ28��������Ϊ14 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
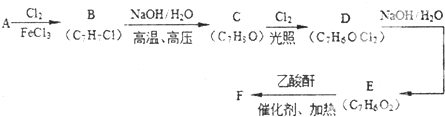
| A��A��B���ڼӳɷ�Ӧ | |||
| B��B��D����������Ʒ�Ӧ | |||
| C������C�Ľṹ��ʽΪCH3CHO | |||
D������E�Ļ�ѧ����ʽΪ��CH3CH2OH+CH3COOH
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
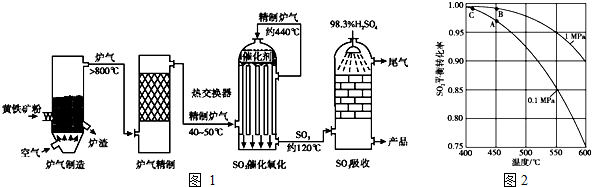
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
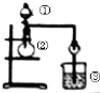
| ѡ�� | �� | �� | �� | ʵ����� |
| A | Ũ���� | MnO2 | NaBr��Һ | ������Cl2��Br2 |
| B | Ũ��ˮ | ��ʯ�� | AgNO3��Һ | AgOH�������� |
| C | Ũ���� | Na2SO3 | FeCl3��Һ | SO2���л�ԭ�� |
| D | ϡ���� | Na2CO3 | Na2SiO3 | �ǽ����ԣ�Cl��Si |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
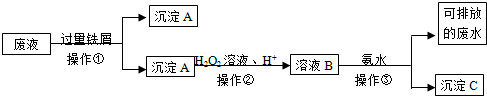
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com