
����Ŀ��ͭ����Ҫ�Ľ������ϡ�
(1)��ҵ�Ͽ���Cu2S��O2��Ӧ��ȡ��ͭ���÷�Ӧ��������Ϊ________������ͭ��ȡ��ͭ�����ʱ������������________�����Һ�б��뺬�е���������________��
(2)��100 mL 18 mol��L��1Ũ�����м��������ͭƬ������ʹ֮��ַ�Ӧ����Ӧ�б���ԭ��H2SO4Ϊ________mol��
(3)���ӹ�ҵ������������Ϊ30%��FeCl3��Һ��ʴ����ͭ���ľ�Ե����ӡˢ��·�壬Ϊ�˴�ʹ�ù��ķϸ�ʴҺ�л���ͭ�������µõ�FeCl3��Һ���������ʵ�����̡�
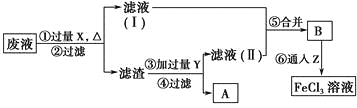
���������У������Լ��Ļ�ѧʽΪ��X________��Y________��Z________����������Ӧ�����ӷ���ʽΪ___________________��
���𰸡�(1) Cu2S��O2 ��ͭ Cu2�� С��0.9 Fe HCl Cl2 2Fe2����Cl2=2Fe3����2Cl��
��������
��1����ҵ�Ͽ���Cu2S��O2��Ӧ��ȡ��ͭʱ��ͭԪ�غ���Ԫ�ػ��ϼ۽��ͱ���ԭ��Cu2S��O2��������������ͭ��ȡ��ͭʱ����ͭ����������ͭ�����������п�����ͭ�ε���Һ���������Һ���ʴ�Ϊ��Cu2S��O2����ͭ��Cu2����
��2��ͭ��Ũ���Ṳ�ȷ�Ӧʱ��Ũ�����ڷ�Ӧ������������������ã����ŷ�Ӧ���У�����Ũ�ȼ�С��ͭ��ϡ�����Ӧ������100 mL 18 mol��L��1Ũ�����м��������ͭƬ������ʹ֮��ַ�Ӧ����Ӧ�б���ԭ��H2SO4С��0.9���ʴ�Ϊ��С��0.9��
��3����30%��FeCl3��Һ��ʴ��ͭ���ľ�Ե��ӡˢ��·��ʱ������Ӧ��2Fe3����Cu=2Fe2����Cu2������Һ�лẬ��Fe2����Cu2����Fe3�����������ۺ�2Fe3����Fe=3Fe2����Fe��Cu2��=Cu��Fe2���������к��й�����Fe��Cu���������Fe��2HCl=FeCl2��H2����ͨ��Cl2��2FeCl2��Cl2=2FeCl3���ʴ�Ϊ��Fe��HCl��Cl2��2Fe2����Cl2=2Fe3����2Cl����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��23��ʱ���������ͬ��������Һ����pH=3�� CH3COOH��Һ��pH=3�������pH=11�İ�ˮ��pH=11��NaOH��Һ������˵������ȷ����
A.������ˮ����ϡ���� ![]() ����
����
B.������ں͢۵���Һ��Ϻ���Һ������
C.����������У�ˮ�ĵ���̶���������С
D.�ۺֱܷ͢��â��кͣ����Ģڵ��������>��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼���Ƶ���;�ܹ㣬������ұ�𡢷�֯��ƯȾ�ȹ�ҵ�Ļ���ԭ�ϣ����������ش��������⣺
��![]() �������繤ҵ����̼���Ƶķ�����·����
�������繤ҵ����̼���Ƶķ�����·����![]() �������������£�
�������������£�

![]() ����I����һ������ ______ �����̢�ķ�Ӧ�ֲ����У�
����I����һ������ ______ �����̢�ķ�Ӧ�ֲ����У�![]()

![]() ��
��
![]() ��ʯ��ʯ�������ֽⷴӦ���ܷ�Ӧ����ʽ�ɱ�ʾΪ ______ ��
��ʯ��ʯ�������ֽⷴӦ���ܷ�Ӧ����ʽ�ɱ�ʾΪ ______ ��
��![]() �꣬����ʱ������ά
�꣬����ʱ������ά![]()
![]() �ð������̼���ƣ���Ӧԭ�����£�
�ð������̼���ƣ���Ӧԭ�����£�

![]() ʱһЩ������ˮ�е��ܽ��
ʱһЩ������ˮ�е��ܽ��![]()
NaCl |
|
|
|
|
|
|
|
|
|
![]() ������ɴ����ԭ���� ______ ����ѭ�����õ������� ______ ��
������ɴ����ԭ���� ______ ����ѭ�����õ������� ______ ��
![]() ����NaCl��Һͨ
����NaCl��Һͨ![]() ��
��![]() ������
������![]() ��ԭ���У� ______ ��
��ԭ���У� ______ ��
��![]() �ҹ�����ר�Һ�°��о��������Ƽ���䷴Ӧԭ���Ͱ�����ƣ������ư����Ƽ����ϣ������ԭ�������ʣ�
�ҹ�����ר�Һ�°��о��������Ƽ���䷴Ӧԭ���Ͱ�����ƣ������ư����Ƽ����ϣ������ԭ�������ʣ�

![]() ��������������
��������������![]() �����õ���Һ�м���NaCl���岢ͨ��
�����õ���Һ�м���NaCl���岢ͨ��![]() ���� ______
���� ______ ![]() ���¶ȷ�Χ
���¶ȷ�Χ![]() ������ ______
������ ______ ![]() �ѧʽ
�ѧʽ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ܸ߷���P�ĺϳ�·�����£�

��1��A�ķ���ʽ��C7H8����ṹ��ʽ��________________________��
��2���Լ�a��____________________��
��3����Ӧ�۵Ļ�ѧ����ʽ��_________________________________________________��
��4��E�ķ���ʽ��C6H10O2��E�к��еĹ����ţ�_________________________��
��5����Ӧ�ܵķ�Ӧ������____________________________��
��6����Ӧ�ݵĻ�ѧ����ʽ��_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ⱦ�����Чȥ������Դ�ij�������ǻ�ѧ�츣�������Ҫ�о����⡣�����������γ����ꡢ�����Ȼ�����Ⱦ��������ף����ú��ʵĴ�ʩ��������Ⱦ�DZ�����������Ҫ��ʩ��
�����о���������NH3���������Ṥҵβ���е�NO��Ⱦ��NH3��NO�����ʵ���֮�ȷֱ�Ϊ1��3��3��l��4��1ʱ��NO�ѳ������¶ȱ仯��������ͼ��ʾ��
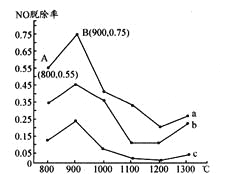
��1��������a�У�NO����ʼŨ��Ϊ6��10��4mg��m3����A�㵽B�㾭��0.8s����ʱ�����NO���ѳ�����Ϊ_____mg��(m3��s)��
������b��Ӧ��NH3��NO�����ʵ���֮����______����������_________��
��2����֪��25����101kPaʱ��
N2(g)+3H2(g) =2NH3(g) ��H��-Q1��mol
2H2(g)+O2(g) =2H2O(1) ��H��- Q2kJ��mo1
N2(g) +O2(g) = 2NO(g) ��H��+ Q3kJ��mo1
��д����NH3�ѳ�NO���Ȼ�ѧ����ʽ__________��
������ҵ�ϻ����Ա�������Ϊ���������������ﺬ�е�SO2��NO����Ⱦ��ת��ΪNa2S2O4(���շ�)��NH4NO3�Ȼ�����Ʒ���������������£�

��3��װ������NOת��ΪNO3���ķ�Ӧ�����ӷ���ʽΪ__________��
��4��װ�����Ƶ�Na2S2O4��ͬʱ������Ce4��������ԭ����ͼ��ʾ���������ĵ缫��ӦʽΪ ��______��
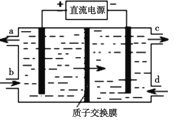
��5�����������̴�������amolSO2��bmolNO������(b>a)������ȡNa2S2O4��NH4NO3��װ������SO2��װ������NO��װ������HSO3����Ce3����װ������NO2��ȫ��ת����������Ϻ�װ������Ce4����ʣ��������������û�б仯������������װ������ͨ���״���µ�O2____L(�ú�a��b�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ķ�����̼�͵�����Ӧ�����ɰ�������粒��壬��ѧ����ʽΪ��2NH3��g��+CO2��g��![]() NH2COONH4��s��
NH2COONH4��s��![]() �����Ȼ�̼��ͨ�������̼�Ͱ��Ʊ���������淋�ʵ��װ����ͼ��ʾ���ش��������⣺
�����Ȼ�̼��ͨ�������̼�Ͱ��Ʊ���������淋�ʵ��װ����ͼ��ʾ���ش��������⣺
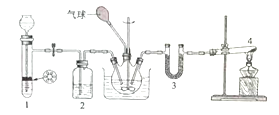
��1��װ��1�����Ʊ�������̼���壺����״ʯ��ʯ�������Թ��еĴ������ϰ��ϣ�©���������Լ�Ϊ____��װ��2�������Լ�Ϊ____��
��2��װ��4���Լ�Ϊ����NH4Cl��Ca��OH��2��������Ӧ�Ļ�ѧ����ʽΪ________���Թܿڲ���������б��ԭ����__________��װ��3���Լ�ΪKOH��������Ϊ______________��
��3����Ӧʱ����ƿ������ˮԡ��ȴ����Ŀ����_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�������ȷ����
A.�����½��еķ�Ӧ�������ȷ�Ӧ���������ܹ����еķ�Ӧ���Ƿ��ȷ�Ӧ
B.ʵ������4mol SO2��2mol O2�������з�Ӧ��2SO2(g)+O2(g)![]() 2SO3(g)��H=-196.64kJ/mol�����ų�314.624kJ����ʱ��SO2��ת����Ϊ80%
2SO3(g)��H=-196.64kJ/mol�����ų�314.624kJ����ʱ��SO2��ת����Ϊ80%
C.�����£���ˮ��ͨ��һ��������������ˮ�ĵ���ƽ�ⱻ�ٽ�
D.��3mL0.1mol/LAgNO3��Һ�е���5��0.1mol/L NaCl��Һ������ɫ�������ٵ���KIϡ��Һ�����Ի�ɫ����Ksp(AgI)��Ksp(AgCl)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ij��ȤС����ݽ̲�ʵ����Ƶ�һ����˵��̼������̼���������ȶ��Ե���ʵ�顣��۲�����ͼ��ʾʵ��װ�ã�����ʵ��ԭ�������ж�����˵�������������в���ѧ����(�� )

A. ��ΪС�մ���Ϊ����
B. Ҫ֤��̼�����������ܲ���ˮ������С�Թ�������մ����ˮ����ͭ��ĩ������
C. ���Ȳ��þ��ܿ���A�ձ��ij���ʯ��ˮ�����
D. ����ʵ������ж�û�з���A�ձ��ij���ʯ��ˮ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ô������ɽ�����β���е�NO��COת���![]() ��
��![]() ����ѧ����ʽ���£�
����ѧ����ʽ���£�![]() ij�¶��£����ݻ�������ܱ�������ͨ��NO��CO����ò�ͬʱ���NO��CO��Ũ�����±�������˵���У�����ȷ����
ij�¶��£����ݻ�������ܱ�������ͨ��NO��CO����ò�ͬʱ���NO��CO��Ũ�����±�������˵���У�����ȷ����
ʱ�� | 0 | 1 | 2 | 3 | 4 | 5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A.2s�ڵ�ƽ����Ӧ����![]()
B.�ڸ��¶��£���Ӧ��ƽ�ⳣ��![]()
C.����NO��CO����ʼͶ�������ӱ�����ƽ��ʱNOת����С��![]()
D.ʹ�ô���������ߵ�λʱ��CO��NO�Ĵ�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com