
����Ŀ������ѧ����ѡ��3�����ʽṹ�����ʡ��绯ѧ��ԭ������һ�ִ���CO2����ɫ�������з�չDZ���ķ�����ͭ��������(In)�Ƚ������Ǹ÷�Ӧ�Ĵ�����
(1)InԪ�ػ�̬ԭ�ӵļ۵����Ų�ʽΪ____________����CuԪ��ͬ���ڣ��һ�̬ԭ����2��δ�ɶԵ��ӵĹ���Ԫ����_________________(��Ԫ�ط���)��
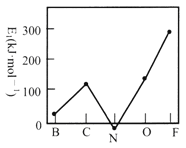
(2)��һ��������(E1)��Ԫ�صĻ�̬��̬ԭ�ӵõ�һ�������γ���̬��һ������ʱ���ų����������ڶ����ڲ���Ԫ�ص�E1�仯������ͼ��ʾ���Է���̼Ԫ�ص�E1�ϴ��ԭ��_____________________________��
(3)[PtC14(NH3)2]��Nԭ�ӵ��ӻ���ʽΪ________��������ѧ�����Ͱ���_______(����ĸ)��
a�����Ӽ�b�����ۼ�c��������d����λ��e�����
[PtCl4(NH3)2]��H-N-H��֮��н�______(�>����<����=��)NH3������H-N-H��֮��нǣ�ԭ����__________________________________��
(4)C60����ɲ�ȡ�����ܶѻ���Ȼ���ڿ�϶�в���������ӻ�ó����壬��ͼΪK3C60������������������K+ռ�ݵ���C60Χ�ɵ����������϶��_____��϶����C60���ӵ�ԭ����������ֱ�ΪA(0��0��0)��B(![]() )��C(1��1��1)�ȣ������Aλ��C60���������K+��ԭ���������Ϊ_____________���жϾ����۷е�ߵͣ�C60_________K3C60(�>����<����=��)��
)��C(1��1��1)�ȣ������Aλ��C60���������K+��ԭ���������Ϊ_____________���жϾ����۷е�ߵͣ�C60_________K3C60(�>����<����=��)��

���𰸡� 5s25p1 TiNi ̼ԭ�ӽ��һ�����Ӻ��2p3���Ϊ�����״̬���ȶ��Խ�ǿ sp3 bd > [PtC14(NH3)2]�γɹ����У�NH3�е�Nԭ�ӵŶԵ�����Pt4+�γ�����λ����ת��Ϊ�ɼ����Ӷԣ��������ɼ����ӶԵij������ͣ����Լ������� �������� ![]() <
<
��������������������⿼��۵����Ų�ʽ����д����һ����������ԭ���ӻ���ʽ���жϡ���ѧ�����жϡ������ķ����������۵�ߵ͵ıȽϡ�
��1��Inλ��Ԫ�����ڱ��е�������IIIA�壬��̬Inԭ�ӵļ۵����Ų�ʽΪ5s25p1��Cu���ڵ������ڣ���Cuͬ�����һ�̬ԭ����2��δ�ɶԵ��ӵĹ���Ԫ����Ti��Ni��
��2����̬̼ԭ�ӵļ۵����Ų�ʽΪ2s22p2��̼ԭ�ӵõ�һ�����Ӻ��2p3���Ϊ�����״̬���ȶ��Խ�ǿ���ų��������ϴ�����̼Ԫ�ص�E1�ϴ���
��3��[PtCl4��NH3��2]��ÿ��Nԭ���γ�3������Ҽ�����Pt4+�γ�1����λ������λ��Ҳ���ڦҼ���Nԭ����û�йµ��Ӷԣ�Nԭ�ӵ��ӻ���ʽΪsp3�ӻ���[PtCl4��NH3��2]��N��H֮���γɹ��ۼ���Pt4+��Cl-��NH3���Ӽ��γ���λ����[PtCl4��NH3��2]������ѧ�����ͣ����ۼ�����λ������ѡbd��[PtCl4��NH3��2]��NH3������Nԭ�Ӷ�����sp3�ӻ���[PtCl4��NH3��2]�γɹ����У�NH3�е�Nԭ���ϵŵ��Ӷ���Pt4+�γ�����λ����ת��Ϊ�ɼ����Ӷԣ��������ɼ����ӶԵij������ͣ����Լ���������[PtCl4��NH3��2]��H-N-H��֮��н�![]() NH3������H-N-H��֮��нǡ�
NH3������H-N-H��֮��нǡ�
��4������ͼʾC60λ�ھ�����8�������6�����ģ��á���̯������1�������к�C60��8![]() +6
+6![]() =4�����ݻ�ѧʽK3C60��1�������к�12��K+������8��K+ռ��C60Χ�ɵ����������϶��4��K+ռ��C60Χ�ɵ����������϶���������϶�����ķֱ������ĺ������ϣ�������Aλ��C60���������K+λ��ͼ
=4�����ݻ�ѧʽK3C60��1�������к�12��K+������8��K+ռ��C60Χ�ɵ����������϶��4��K+ռ��C60Χ�ɵ����������϶���������϶�����ķֱ������ĺ������ϣ�������Aλ��C60���������K+λ��ͼ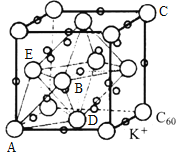 ��ABDEΧ�ɵ����������϶����C60���ӵ�ԭ����������ֱ�ΪA��0��0��0����B��
��ABDEΧ�ɵ����������϶����C60���ӵ�ԭ����������ֱ�ΪA��0��0��0����B��![]() ��0��
��0��![]() ����C��1��1��1���ȣ���ԭ���������D��
����C��1��1��1���ȣ���ԭ���������D��![]() ��
��![]() ��0����E��0��
��0����E��0��![]() ��
��![]() ��������Aλ��C60���������K+��ԭ���������Ϊ��
��������Aλ��C60���������K+��ԭ���������Ϊ��![]() ��
��![]() ��
��![]() ����C60���ڷ��Ӿ��壬K3C60�������Ӿ��壬���Ӽ�������С�����Ӽ��������۷е�ߵͣ�C60
����C60���ڷ��Ӿ��壬K3C60�������Ӿ��壬���Ӽ�������С�����Ӽ��������۷е�ߵͣ�C60![]() K3C60��
K3C60��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�ij��ȤС������ͼװ����ͨ����н�������ʵ�飺
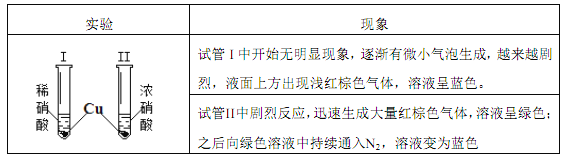
����˵����ȷ����
A. �Թ� I ��dz����ɫ����Ϊ NO2�������ỹԭ����
B. �������� Cu ��ȫ�ܽ�ʱ�� I �����ĵ� HNO3 ����
C. ���� Fe ֮���ظ�ʵ�飬��Ȼ���Թ� II �з�Ӧ������
D. �Թ� II �з�Ӧ����Һ��ɫ���Թ� I �еIJ�ͬ�� ���������� NO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A. 1mol��OH��10NA������
B. 1molC2H518OH������CH3COOH��ַ�Ӧ�ɵ�NA��H2O
C. ��״���£�11.2L��ϩ�����ļ��Թ��ۼ���Ϊ3NA
D. �����£���1mol�����뵽������Ũ�����У�����NO2������������67.2L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2017��3��21���ǵڶ�ʮ���������ˮ����������ˮ��Դ���������÷�ˮ��ʡˮ��Դ����ǿ��ˮ�Ļ��������ѱ�Խ��Խ���������ע����֪��ij��ɫ��ˮ�п��ܺ���H����NH4+��Fe3����Al3����Mg2����Na����NO3-��CO32-��SO42-�еļ��֣�Ϊ������ɷ֣��ֱ�ȡ��ˮ��Ʒ1L������������ʵ�飬��������й�ͼ��������ʾ��

��ش��������⣺
(1)��������3��ʵ����Է�����ˮ��һ�������ڵ���������__________��һ�����ڵ���������___________��
(2)д��ʵ���ͼ���г����ﵽ��������������ٷ����仯�η�����Ӧ�����ӷ�Ӧ����ʽ��________��
(3)����ͼ����ԭ��Һ��c(NH4+)��c(Al3��)�ı�ֵΪ______�����ó��������������______g��
(4)��ͨ��ʵ��ȷ��ԭ��ˮ��c(Na��)=0.18 mol��L-1,���ж�ԭ��ˮ��NO3-�Ƿ���ڣ�____(��������������������������ȷ����)�������ڣ� c(NO3-) = _____ mol��L-1��(�������ڻ�ȷ����˿ղ���)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ʻ�ѧ����й������ں�������ѧ�������ǵ�������ǵ�δ������
��1���Ա���ù�����������������д�����������������̼��Ӧ�Ļ�ѧ����ʽ��__________________________��
��2��С�մ���������ķ��ݼ���д��С�մ�����ˮ�ĵ��뷽��ʽ��_________________��
��3�����ӹ�ҵ����30%��FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭ��������ӡˢ��·�塣д��FeCl3��Һ�����ͭ������Ӧ�����ӷ���ʽ��_________________________________��
��4����ȥNa2CO3��ĩ�л����NaHCO3������ѷ�����_________����ѧ����ʽΪ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�����������ͼ��ʾʵ��װ�����ش��������⡣
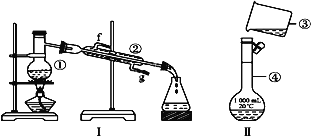
��1��д���������������ƣ���______________����_____________����____________��
��2��������~���У�ʹ��ʱ�������Ƿ�©ˮ����____________(�����)��
��3��������ƾ���ˮ�Ļ������뷽��Ϊ___________���÷��������ڷ���________��ͬ�Ļ���������װ��������ƾ���ˮ�Ļ��������ȱ�ٵ�������_____________���ڵĽ�ˮ����____________(����f������g��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Na2O2��Na2O������˵����ȷ����
A. �������Ӹ����Ⱦ�Ϊ1��2
B. ���ǽ��������������ȷ�Ӧ�Ƶ�
C. ��ˮ��Ӧ������ͬ
D. Na2O2��Na2O�������ڿ����У����ղ��ﲻͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������һ�ִ��ж�ṹ��ϸС������ɵ������Ͻ𣬱��㷺�����л�����⻯��Ӧ�Ĵ������Ժ�������(��Ҫ�ɷ�ΪNiS��FeS��SiO2��)Ϊԭ���Ʊ��������Ĺ�����������ͼ��ʾ��
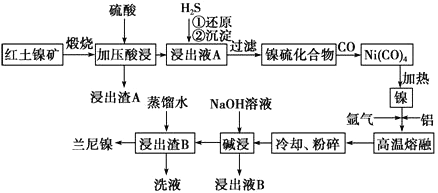
��1��������A����Ҫ�ɷ���_______________���ѧʽ����
��2����֪�����������պ�����Ni2O3������ѹ��������ҺA�к��д���Ni2����д���й���Ԫ�صļ�ѹ����Ļ�ѧ��Ӧ����ʽ_______________________________��
��3�������ҺA��ͨ��H2S���壬�ٻ�ԭ���������漰��Ҫ��Ӧ�����ӷ���ʽ��__________________________________��
��4�����γ�Ni(CO)4�Ĺ����У�̼Ԫ�صĻ��ϼ�û�б仯����Ni(CO)4�е�Ni�Ļ��ϼ�Ϊ________�� ��5�����������Ŀ����ʹ��������ṹ���Ӷ���ǿ��������ǿ�����ԣ��˹����з�����Ӧ�����ӷ���ʽΪ__________________________��
��6������ʱ����Ũ�Ⱦ�Ϊ1.0mol��L-1��FeSO4��NiSO4�Ļ����Һ�еμ�Na2S ���壬��Ni2+ǡ�ó�����ȫʱ��������Һ��c(Fe2+)=______________��
����֪����25�棬Ksp(NiS)=2.0��10-21��Ksp(FeS)=6.0��10-18
����Һ�е�����Ũ�ȡ�10-5 mol��L-1ʱ����Ϊ�����ӳ�����ȫ����
��7������ҺB���Ի��գ������������Ա�ѭ�����á�����ƼĻ������̣�
����ҺB��___________________________________________________________�� ����ͷ��ע����ӷ�Ӧ��Ļ�ѧʽ�ͷ�Ӧ��������(ʾ����![]() )
)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ѧ-ѡ��5:�л���ѧ������������̹��һ�����Ʒζ�����Ѫѹҩ��,�ɷ��㻯����A�ϳ����м���G��һ�ֺϳ�·������:
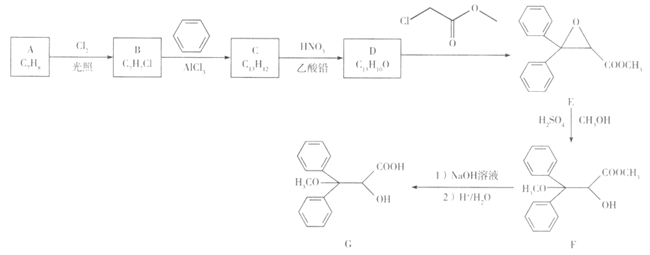
��֪:C�ĺ˴Ź�������ֻ��4 ��壬Dֻ��3��塣
�ش���������:
(1)A�Ļ�ѧ������_______��G�к��������ŵ�������______��
(2)C�Ľṹ��ʽ��______��D�Ľṹ��ʽ��______ ��
(3)B��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��____________��
(4)�������������ķ��㻯����X��E��ͬ���칹�壬X������������ˮ��ֻ����һ���л����X��____�֣����к˴Ź���������3���,�����֮��Ϊ3:2:2�Ľṹ��ʽΪ______ ��
(5)д���ü���ͱ�Ϊԭ���Ʊ�������2��4��6-������������ĺϳ�·��: ______(�����Լ���ѡ)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com