
����Ŀ������˵����ȷ���ǣ� ��
A.101kPaʱ��2H2(g)+O2(g)=2H2O(l) ��H=-572kJ��mol-1����H2��ȼ������H=-572kJ��mol-1
B.һ�������·�����Ӧ��N2(g)+3H2(g)![]() 2NH3(g) ��H=-92.4kJ��mol-1���������½�1.5mol H2����N2��ַ�Ӧ���ų�����46.2kJ
2NH3(g) ��H=-92.4kJ��mol-1���������½�1.5mol H2����N2��ַ�Ӧ���ų�����46.2kJ
C.�������������������������ֱ���ȫȼ�գ����߷ų�������
D.��֪��2C(s)+2O2(g)=2CO2(g) ��H1��2C(s)+O2(g)=2CO(g) ��H2������H1����H2
���𰸡�D
��������
A��ȼ������1mol��ȼ����ȫȼ�������ȶ���������ʱ�ų�����������572kJ��2mol����ȼ�շų���������A����
B��N2(g)+3H2(g)![]() 2NH3(g)�ǿ��淴Ӧ�����ܽ��е��ף��ų���������С��46.2kJ��B����
2NH3(g)�ǿ��淴Ӧ�����ܽ��е��ף��ų���������С��46.2kJ��B����
C����̬��ȹ�̬�������ߣ�ȼ�շ��ȶ࣬C����
D��������̼��ȫȼ�����ɶ�����̼�ų����������ڲ���ȫȼ������CO�ų�������������HΪ��ֵ��������H1����H2��D��ȷ��
��ѡD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ӱ�컯ѧ��Ӧ���ʵ����غܶ࣬ij������ȤС����ʵ�鷽������̽����
(1)ȡ�����ʵ���Ũ�ȡ��������H2O2��Һ�ֱ����H2O2�ķֽ�ʵ�飬ʵ�鱨�����±���ʾ������ͽ����ԣ���

��ʵ��1��2�о�����__________��H2O2�ֽ����ʵ�Ӱ�졣
��ʵ��2��3��Ŀ����_______________��H2O2�ֽ����ʵ�Ӱ�졣
(2)������֪��Cu2����H2O2�ֽ�Ҳ�д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч������С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺

�ٶ�����ͼ��ͨ���۲�_______�����ԱȽϵó����ۡ���ͬѧ�����CuSO4��Һ��ΪCuCl2��Һ����������������_________��
�ڶ�����ͼ����ʾ��ʵ��ʱ���ռ���40 mL����Ϊ��������������Ӱ��ʵ������أ�ʵ������Ҫ������������__________��
(3)���Ը��������Һ�Ͳ�����Һ�ɷ�����Ӧ��2KMnO4��5H2C2O4��3H2SO4=K2SO4��2MnSO4��8H2O��10CO2����ʵ��ʱ���ֿ�ʼ��Ӧ���ʽ�������Һ��ɫ�����ԣ���һ��ʱ���ͻȻ��ɫ����Ӧ�������Լӿ졣�Դ�չ�����ۣ�
��ijͬѧ��ΪKMnO4��H2C2O4�ķ�Ӧ��______�ȷ�Ӧ������_______________��
�ڴ�Ӱ�컯ѧ��Ӧ���ʵ����ؿ�������Ϊ��������________��Ӱ�졣Ҫ֤����IJ��룬ʵ�鷽����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�ظ����(K2Cr2O7)����ǿ�����ԣ��仹ԭ����Cr3+��ˮ��Һ�г���ɫ������ɫ����K2Cr2O7��Һ�д�������ƽ�⣺Cr2O72- (��ɫ)+H2O![]() 2CrO42- (��ɫ)+2H+����K2Cr2O7��Һ����ʵ�飬���ʵ�飬����˵����ȷ����
2CrO42- (��ɫ)+2H+����K2Cr2O7��Һ����ʵ�飬���ʵ�飬����˵����ȷ����
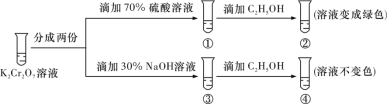
A.������Һ��ɫ���������Һ���
B.����Cr2O72-��C2H5OH����
C.�ԱȢںܿ͢�֪K2Cr2O7������Һ������ǿ
D.������м���70% H2SO4��Һ����������Һ��Ϊ��ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ��Ϊԭ�Ͽ��Ժϳɶ��ָ߾���ĺϳ�·�����£�
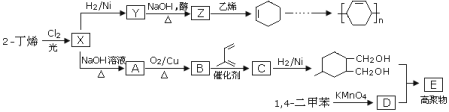
��֪��ϩ����X2��һ���������ܷ���ȡ�������ܷ���˫ϩ�ϳ���![]() ��
��
��ش��������⣺
(1)X�к��еĹ�����Ϊ_____________________________________��
(2)Y��Z�Ļ�ѧ����ʽΪ_____________________________��
(3)�߾���E�Ľṹ��ʽΪ____________________________������A��һ��ͬ���칹�壬����ʵ��ת����![]() ��������Ϊ________��
��������Ϊ________��
(4)��![]() ���Ժϳ�
���Ժϳ�![]() �����ϳ�·�ߵ�˳���漰��Ӧ�ķ�Ӧ�����У�______________��
�����ϳ�·�ߵ�˳���漰��Ӧ�ķ�Ӧ�����У�______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�˲ⶨ���ᾧ��H2C2O4��xH2O�е�xֵ��ijʵ��С�����ʵ�飬�������£�
�ٳ�ȡ1.260 g���ᾧ�壬���100 mL��Һ��
��ȡ25.00 mL��H2C2O4��Һ������ƿ�ڣ��ټ�������ϡ���ᡣ
����Ũ��Ϊ0.1000 mol/L��KMnO4��Һ�ζ�H2C2O4��Һ����__________________ʱ���ζ�������
�ܼ�¼���ݣ��ظ�ʵ�顣���������磺
ʵ����� | V(KMnO4��Һ) | |
�ζ�ǰ�̶�/mL | �ζ���̶�/mL | |
1 | 0.10 | 10.00 |
2 | 1.10 | 11.10 |
3 | 1.50 | 11.50 |
�ش��������⣺
(1)�������ʹ���ձ�����Ͳ������������ȱ�ٵIJ�������Ϊ______________(������)������۵ζ������У�ʢװKMnO4��Һ������Ϊ_______________(������)��
(2)�÷�Ӧԭ���Ļ�ѧ����ʽΪ_____________________________________________��
(3)�뽫����۲�������_____________________________________________________��
(4)�������ݣ�����H2C2O4��Һ�����ʵ���Ũ��Ϊ_________mol/L��x=________��
(5)���ζ��յ����ʱ����KMnO4��ҺҺ�棬��xֵ��_________(�ƫ��ƫС������Ӱ�족)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�ⶨCO2����Է���������ijʵ��С����λͬѧѡ�ú�NaHCO3����Ʒ(������Ϊm1g)�������������Լ�����������������ʵ�顣���������գ�
����������ȷ��CO2��������װ������ͼ��
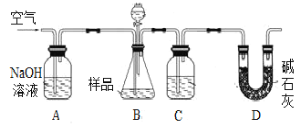
(1)B�з�Ӧ�Ļ�ѧ����ʽΪ____________________________________________________��
(2)ʵ���г�������ͨ�������������֮һ�ǰ����ɵ�CO2ȫ���������װ���У�ʹ֮��ȫ�����գ���������Ϊ___________________________________________________________��
(3)������߲ⶨ��ȷ�ȵĴ�ʩ��___________��
a����B�ڼ�����֮ǰ���ž�װ���ڵ�CO2����
b����B�ڵμ���ʱ���˹���
c����B��C֮������ʢ�б���NaHCO3��Һ��ϴ��װ��
d����D������ʢ�м�ʯ�ҵĸ����
���õζ���ȷ��CO2�����ʵ���������Ʒ���Ƴ�100mL��Һ������ȡ��20.00 mL����c mol��L��1������ζ�(������ָʾ��)����______________________________________________________ʱ��ֹͣ�ζ���ƽ�вⶨ���Σ��й�ʵ�����ݼ�¼���±���m1 g��Ʒ����CO2�����ʵ���Ϊ_____________��
ʵ���� | ����Һ��� (mL) | �����������(mL) | |
������ | ĩ���� | ||
1 | 20.00 | 0.00 | 25.02 |
2 | 20.00 | 0.20 | 28.80 |
3 | 20.00 | 1.30 | 26.28 |
�������������ȷ��CO2�������װ����ͼ��ʾ��

(4)Ϊ�˼�Сʵ�����������м����Һ��XΪ___________________��Һ��
(5)����װ�����������ã�����ƽ�ӣ�����õ���CO2�����������ȻƫС����ԭ�������____________________________________________________________________________��
(6)ȷ��CO2����Է���������ѡ��___________________(��������������������������������д)��ʵ������Ϊ��ѡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶���2L�ܱ������У�3��������ʼ״̬��ƽ��״̬ʱ�����ʵ�����n�����±���ʾ������˵����ȷ���ǣ� ��
X | Y | W | |
n����ʼ״̬��/mol | 2 | 1 | 0 |
n��ƽ��״̬��/mol | 1 | 0.5 | 1.5 |
A.���¶��´�ƽ�������ѹǿƽ�ⲻ�ƶ�
B.�÷�Ӧ����ʽ�ɱ�ʾΪ��X+2Y=3W
C.�����¶ȣ���W�����������С����˷�Ӧ��H��0
D.���º���ʱ������X�����ʵ�����ƽ���������ƶ���X��ת�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����
A.��֪t1��ʱ����ӦC+CO2![]() 2CO ��H>0������Ϊ�����������¶ȣ��淴Ӧ���ʼ�С
2CO ��H>0������Ϊ�����������¶ȣ��淴Ӧ���ʼ�С
B.��ѹ�����з�����ӦN2+O2![]() 2NO�����������г���He�����淴Ӧ���ʾ�����
2NO�����������г���He�����淴Ӧ���ʾ�����
C.��һ������п�ۺ�����6molL1���ᷴӦʱ��Ϊ�˼�����Ӧ����,�ֲ�Ӱ�����H2������,����Ӧ���м���������CuSO4��Һ
D.����ӦPCl5(g)![]() PCl3(g)��Cl2(g) �ﵽƽ������¶Ⱥ�������䣬�ٳ�PCl5(g)�ﵽ�µ�ƽ�⣬��ƽ���ԭƽ�����PCl5(g)��ת���ʼ���
PCl3(g)��Cl2(g) �ﵽƽ������¶Ⱥ�������䣬�ٳ�PCl5(g)�ﵽ�µ�ƽ�⣬��ƽ���ԭƽ�����PCl5(g)��ת���ʼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ij���淴ӦmA(g)+nB(g)![]() pC(g)���ܱ������н��У���ͼ��ʾ�ڲ�ͬ��Ӧʱ��tʱ���¶�T��ѹǿp�뷴Ӧ��B�ڻ�������е��������[��(B)]�Ĺ�ϵ���ߣ������߷����������ж���ȷ����(�� ��)
pC(g)���ܱ������н��У���ͼ��ʾ�ڲ�ͬ��Ӧʱ��tʱ���¶�T��ѹǿp�뷴Ӧ��B�ڻ�������е��������[��(B)]�Ĺ�ϵ���ߣ������߷����������ж���ȷ����(�� ��)
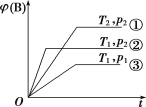
A.T1<T2��p1>p2��m+n>p�����ȷ�Ӧ
B.T1<T2��p1>p2��m+n<p�����ȷ�Ӧ
C.T1>T2��p1<p2��m+n<p�����ȷ�Ӧ
D.T1>T2��p1<p2��m+n>p�����ȷ�Ӧ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com