
����Ŀ����ȩ(HCHO)�׳���ȩ����һ����Ҫ�Ļ���ԭ�ϡ���ͨ�����·������״�ת��Ϊ��ȩ��
���ⷨ��CH3OH(g)=HCHO(g)��H2(g) ��H1����92.09 kJ��mol��1
��������CH3OH(g)��![]() O2(g)=HCHO(g)��H2O(g)��H2
O2(g)=HCHO(g)��H2O(g)��H2
�ش���������:
(1)��֪��2H2(g)��O2(g)=2H2O(g)��H3����483.64 kJ��mol��1������H2��_________________��
(2)�����ⷨ��ȣ�������������ѧ�����ƽϴ���ԭ��Ϊ________________________________________��
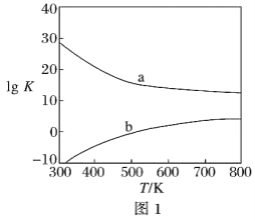
(3)ͼ1Ϊ�״��Ʊ���ȩ��Ӧ��lg K(KΪƽ�ⳣ��)���¶�(T)�ı仯���ߡ�����_____(����a������b��)��Ӧ���ⷨ���ж�������_____________________________________��
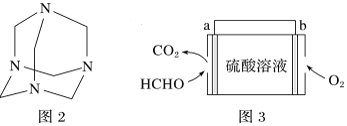
(4)����ȩˮ��Һ�백ˮ����������Ƶ�������Ʒ(�ṹ��ʽ��ͼ2)����������ҽҩ�ȹ�ҵ���й㷺��;����ԭ����ȫ��Ӧ����������Ʒ�����ȩ�백�����ʵ���֮��Ϊ___________��
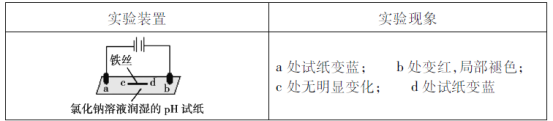
(5)���ڼ�ȩ�����Σ�����彡����ͨ�����������Լ������м�ȩ�ĺ�����һ��ȼ�ϵ���ͼ�ȩ���崫������ԭ����ͼ3��ʾ����a���ĵ缫��ӦʽΪ_________________________________________________������·��ת��4��10��4 mol����ʱ���������ڲμӷ�Ӧ��HCHOΪ________________mg��
���𰸡���149.73 kJ��mol��1 ���ⷨ�ķ�ӦΪ���ȷ�Ӧ���������ķ�ӦΪ���ȷ�Ӧ�����ȷ�Ӧ������ѧ�����ƽϴ� b ���ⷨΪ���ȷ�Ӧ���¶����ߣ�K���� 3��2 HCHO��H2O -4e�� = CO2��4H�� 3
��������
(1)��֪i��CH3OH(g)�THCHO(g)+H2(g)��H1=+92.09kJmol-1
ii.2H2(g)+O2(g)�T2H2O(g)��H3=-483.64kJmol-1��
���ݸ�˹����i+![]() ii�÷���ʽCH3OH(g)+
ii�÷���ʽCH3OH(g)+![]() O2(g)=HCHO(g)+H2O(g)��H2=(+92.09-
O2(g)=HCHO(g)+H2O(g)��H2=(+92.09-![]() ��483.64)kJ/mol=-149.73kJmol-1��
��483.64)kJ/mol=-149.73kJmol-1��
(2)�����Ȼ�ѧ��Ӧ����ʽ��֪���ⷨ�ʱ������Ϊ���ȷ�Ӧ���������ʱ�С����Ϊ���ȷ�Ӧ�����ȷ�Ӧ������ѧ�����ƽϴ�
(3)���ⷨΪ���ȷ�Ӧ���¶����ߣ�K������������b��Ӧ�������ⷨ��
(4)����ȩˮ��Һ�백ˮ����������Ƶ�������Ʒ����ԭ����ȫ��Ӧ����������Ʒ��ÿ��������Ʒ�����к���6��Cԭ�ӡ�4��Nԭ�ӣ�ÿ����ȩ�����к���1��Cԭ�ӡ�ÿ�����������к���1��Nԭ�ӣ�����Cԭ�ӡ�Nԭ���غ�֪��Ҫ�γ�һ��������Ʒ������Ҫ6����ȩ���ӡ�4���������ӣ�����Ҫ��ȩ�Ͱ������Ӹ���֮��=6��4=3��2�������ʵ���֮��Ϊ3:2��
(5)��ͼ��֪a��HCHO����������CO2����aΪԭ��ظ�����ʧ���ӷ���������Ӧ���缫����ʽΪHCHO��H2O -4e�� = CO2��4H�������ݵ缫����ʽ��֪ת��4��10��4 mol����ʱ�����뷴Ӧ��HCHOΪ1��10��4mol������Ϊ1��10��4mol��30g/mol=0.003g=3mg��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʯī�缫������е��ʵ�顣
���ж�ʵ������Ľ��ͻ��Ʋⲻ��������
A.aΪ���ص�����
B.b�����������ɣ�����ˮ��Ӧ����������ʹ�����
C.c�������˷�Ӧ��Fe��3e����Fe3��
D.d����2H2O��2e����H2����2OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����õ�ⷨ��ȡ Na2FeO4 ��װ��ͼ��ͼ��ʾ������˵����ȷ���ǣ����������¶ȱ� �ֲ��䣬��Һ����仯���Բ��ƣ�
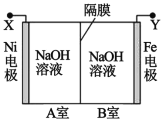
A.Y ����ӵ�Դ��������Fe �缫�Ϸ�����ԭ��Ӧ
B.Ni �缫�Ϸ����ĵ缫��ӦΪ��2H2O �� 4e��== O2����4H��
C.����ĤΪ�����ӽ���Ĥ����������� OH���� B �ҽ��� A ��
D.����ȥ��Ĥ����ֻ�ϣ����Һ�� pH ��ԭ��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ���У��ܴﵽ��Ӧʵ��Ŀ�ĵ���
|
|
|
|
A���Ʊ����ռ��������� | B��֤���Ȼ����ܽ�ȴ������� | C����֤���������ȥ��������ϩ | D���ƶ�S��C��Si�ķǽ�����ǿ�� |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ����0.10mol��L-1��H2C2O4(��Ԫ����)��Һ�еμ�NaOH��Һ����Һ�в����������ʵ���Ũ����pH�ı仯������ͼ��ʾ������˵������ȷ����

A. 25��ʱH2C2O4��һ�����볣��ΪKa1=104.3
B. pH=2.7����Һ�У�c(H2C2O4)=c(C2O42-)
C. pH=7����Һ�У�c(Na+)>2c(C2O42-)
D. �μ�NaOH��Һ�Ĺ�����ʼ�մ��ڣ�c(OH-)+2c(C2O42-)+c(HC2O4-)=c(Na+)+c(H+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ķ����ռ���ʵ��������������������������Դ������Ҫ��ʩ������������ͼ��ʾ��־���������ж���������Ӧ��

A.��������B.�ɻ�������C.�к�����D.��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ִ������н����벻�����ֲ��ϡ�
(1)ˮ�����________�Ժ�ǿ��ˮ�ԣ�ˮ��ɰ����________��________��ˮ�Ļ����ǽ������ϼ�����������________��________��________�Ļ����øֽ����ṹ�Ļ�����ǿ�Ⱥܴ�
(2)������������Ҫԭ����________��________��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij������ӵ�ص����������ǽ���������ﮣ�LiCoO2���������۾���Ϳ�����������Ƴɵģ������������ã�ijУ�о�С�鳢�Ի��շϾ����������е��ܡ�
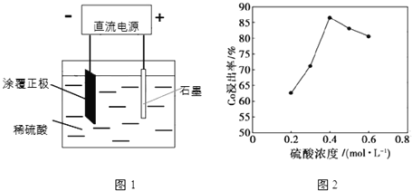
(1)25��ʱ����ͼ1��ʾװ�ý��е�⣬��һ����������Co2+����ʽ���������н������������������ݲ�����һ��ʱ������������������롣
�������ĵ缫��ӦʽΪ��LiCoO2+4H++e-=Li++Co2++2H2O�������ĵ缫��ӦʽΪ______��
�ڸ��о�С�鷢������Ũ�ȶ��ܵĽ������нϴ�Ӱ�죬һ�������£������仯������ͼ2��ʾ����c��H2SO4����0.4molL-1ʱ���ܵĽ������½�����ԭ�����Ϊ______��
(2)�����ɺ�õ���Co2+�Ľ���Һ���������������۳����ڵ��۵ײ��������²�����������ܡ�

��д��������������������۷�����Ӧ�Ļ�ѧ����ʽ______���ò���һ����80�����½��У��¶Ȳ���̫�ߵ�ԭ����______��
����֪��NH4��2C2O4��Һ�������ԣ����й�ϵ����ȷ����______������ĸ��ţ���
a��c(NH4+)��c(C2O42-)��c(H+)��c(OH-)
b��c(H+)+c(NH4+)=c(OH-)+c(HC2O4-)+c(HC2O42-)
c��c(NH4+)+c(NH3H2O)=2[c(HC2O42-)+c(HC2O4-)+c(H2C2O4)]
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com