
����Ŀ��ʵ����������ͼװ�ý����к��ȵIJⶨ���ش��������⣺
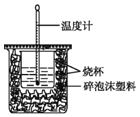
(1)��ͼ����һ��δ����������_______����������)��
(2)�ڲ�����ȷ��ǰ��������к��Ȳⶨ��ȷ�ԵĹؼ���____��
(3)�����0.50 mol/L��������������ƹ������ʵ�飬����ݴ�ʵ���������д�к��ȵ��Ȼ�ѧ����ʽ�е�H��____(����ƫ������ƫС������������)��ԭ����_______��
(4)����50 mL 0.50 mol/L��ϡ������50 mL 0.55 mol/L��ϡ����������Һ����ʵ�飬����Һ���ܶȾ�Ϊ1 g/cm3���кͺ���Һ�ı�����c=4.18 J/(g����)�������ʵ�����ݼ����к���H= __ (ȡС�����һλ)��
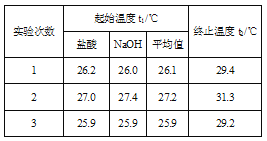
(5)����(4)�еĽ�����к��ȵ�����ֵ��ƫ�����ƫ���ԭ�������___��
a.ʵ��װ�ñ��¡�����Ч����
b.����ȡNaOH��Һ�����ʱ���Ӷ���
c.�ֶ�ΰ�NaOH��Һ����ʢ��ϡ�����С�ձ���
d.���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳⶨ������Һ���¶�
���𰸡����β�������� ��������ɢʧ ƫС NaOH�����ܽ���� -55.2 kJ��mol-1 acd
��������
(1)�ⶨ�к��ȣ�Ҫ�û��β�����������裻
(2)�к��Ȳⶨ��ȷ�ⶨ�����仯��
(3)�������ƹ��ܽ���ȣ�
(4)���ݹ�ʽQ=cm��T���������0.025mol��ˮ�ų��������������к��ȵĸ����������1molˮ�ų���������
��5��55.2С���к���57.3��˵��ʵ���������������ʧ��
(1) �ⶨ�к��ȣ�Ҫ�û��β�����������裬��ͼδ�������β����������
(2)�к��Ȳⶨ��ȷ�ⶨ�����仯������к��Ȳⶨ��ȷ�ԵĹؼ��Ǽ�������ɢʧ��
(3) NaOH�����ܽ���ȣ������0.50 mol/L��������������ƹ������ʵ�飬�����ƫ�࣬���ݴ�ʵ���������д�к��ȵ��Ȼ�ѧ����ʽ�е�H��ƫС��
(4) 3�η�Ӧǰ���¶Ȳ�ֱ�Ϊ��3.3����4.1����3.3�����ڶ�����ȥ��ƽ��ֵΪ3.3����50 mL 0.50 mol/L��ϡ������50 mL 0.55 mol/L��ϡ����������Һ��������m=100mL��1g/mL=100g��c=4.18J/��g���������빫ʽQ=cm��T������0.025mol��ˮ�ų�����Q=4.18J/��g������100g��3.3��=1.3794kJ��������0.025mol��ˮ�ų�����1.3794kJ����������1mol��ˮ�ų�����Ϊ1.3794kJ��40=55.2kJ������ʵ���õ��к�����H=-55.2kJ/mol��
��5��a��װ�ñ��¡�����Ч���������ʧ��õ�����ƫС���к��ȵ���ֵƫС����ѡa��
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ��к��ȵ���ֵƫ�ʲ�ѡb��
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�������ʧ��õ�����ƫС���к��ȵ���ֵƫС����ѡc��
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨHCl��Һ���¶ȣ�HCl��Һ����ʼ�¶�ƫ�ߣ���õ�����ƫС���к��ȵ���ֵƫС����ѡd����Ϊacd��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2019��3�£��ҹ���ѧ���з���һ�����͵�п�ⵥҺ����أ���ԭ����ͼ��ʾ������˵������ȷ����

A. �ŵ�ʱB�缫��ӦʽΪ��I2+2e-=2I-
B. �ŵ�ʱ����ʴ�����������Ũ������
C. MΪ�����ӽ���Ĥ��NΪ�����ӽ���Ĥ
D. ���ʱ��A������65gʱ��C������������Ϊ4NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������⣺(����д���������)
��1����ij�¶��£���H2��I2��0.10mol����̬��������10L���ܱ�������,��ַ�Ӧ���ﵽƽ����c(H2)=0.0080mol/L
����÷�Ӧ��ƽ�ⳣ����_____
���������¶��£�����������ͨ��H2��I2������0.20mol������ﵽ��ѧƽ��״̬ʱ�����ʵ�Ũ�ȡ�____
��2������N2H4���£���NO2��ȼ�գ�����N2��Һ̬H2O���ǻ�����䳣�õķ�Ӧ֮һ����֪��N2(g)+2O2(g)=2NO2(g) ��H1=+67.2kJ/mol��N2H4(g)+O2(g)=N2(g)+2H2O(l) ��H2=-534kJ/mol�����綼����ͬ״̬�£���д����������Ӧ���Ȼ�ѧ����ʽ������д�����̣�____
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��������������ҩ��������[KAl(SO4)2��12H2O]�ֳ�����.���������������ȣ���һ����Ҫ�Ļ�ѧ�Լ�������˵����ȷ���ǣ� ��
A.������ҩ�ﲻ����θҩ�W������̼�����ƽ���ͬʱ����
B.0.1molL-1����Һ��ȫˮ������Al(OH)3������С��6.02��1022
C.��0.1mol������Һ�е���Ba(OH)2��Һ����SO42-��Al3+ȫ��ת��ΪBaSO4��Al(OH)3���������ʱ���ɳ������������
D.�����£�0.1molL-1����Һ��ˮ�����c(H+)С��10-7mol��L-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��Ӧ4CO��2NO2![]() N2��4CO2�ڲ�ͬ�����µĻ�ѧ��Ӧ�������£����б�ʾ��Ӧ���������ǣ� ��
N2��4CO2�ڲ�ͬ�����µĻ�ѧ��Ӧ�������£����б�ʾ��Ӧ���������ǣ� ��
A. ��(CO)��1.5 mol��L��1��min��1 B. ��(NO2)��0.7 mol��L��1��min��1
C. ��(N2)��0.4 mol��L��1��min��1 D. ��(CO2)��1.1 mol��L��1��min��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ˮϡ��0. 1mol/L��CH3COOH��Һʱ��ʼ�ձ����������Ƶ���
A. c(CH3COOH)B. c(H+)C. c(CH3COO��)D. c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����������ͼװ��̽��SO2��ԭCuO������һ�������ﺬ������֪Cu2O+2H+=Cu+Cu2++H2O���ش�����������⣺
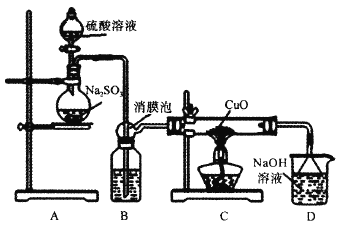
��.SO2��ԭCuO��̽��
��1��װ��B���˷�����Լ�Ϊ___����Ĥ�ݵ�������__��
��2��ʹ��98%��H2SO4��Һ�Ʊ�SO2����������С��ʹ��65����H2SO4��Һ�Ʊ�SO2��ԭ����__��
��3����ַ�Ӧ��ɫ�����Ϊ��ɫ��ȡC�������IJ����ˮ����Һ����ɫ���к�ɫ�����ȡ��ɫ������μ����ᣬ��Һ�ֳ���ɫ������������ɫ������ɴ˿��Եó�SO2��CuO��Ӧ�ķ���ʽΪ__��
��.��������CuSO4�������
��4���������������ⶨ������CuSO4������ȡmg�����ܽ���ˮ����Ϊ250mL��Һ��ȡ20.00mL��Һ�μӼ���ϡ���ᣬ�ټ������KI��Һ���Ե���Ϊָʾ����Na2S2O3����Һ�ζ�����ػ�ѧ��ӦΪ2Cu2++4I-=2CuI��+I2��I2+I-![]() I3-��I2+2S2O32-=S4O62-+2I-��
I3-��I2+2S2O32-=S4O62-+2I-��
��Na2S2O3����Һ�ױ��ʣ��ζ�ǰ��Ҫ�궨�����Ƹ���Һʱ��Ҫ�IJ����������ձ���___�����������Լ�ƿ��
��������0.1000mol/LNa2S2O3����ҺVmL���������CuSO4��������Ϊ____(д����ʽ)��
��CuI�������I3-����ǿ�������������ɴ˻����CuSO4���������ⶨֵ____(����ƫ��������ƫС��)��Ϊ����ʵ�����ζ������У������ڽӽ��յ�ʱ����KSCN��ʹCuIת��Ϊ�ܽ�ȸ�С��CuSCN���ó������I3-��������������KSCN����̫�磬I2��Ũ�Ƚϴ�I2�ὫSCN-��������SO42-��ICN���÷�Ӧ�����ӷ���ʽΪ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A.�����ܶ�С��ˮ������Ϊ����ˮ���ӵ�������·��Ӽ���ֽϴ��϶
B.�⾧���Ƿ��Ӿ��壬��������������ֻ��˷�ԭ�Ӽ�������
C.ij����������̬ʱ�ܵ��磬�������һ�������ӻ�����
D.��![]() ��
��![]() �����У��������������Ӹ����Ⱦ�Ϊ
�����У��������������Ӹ����Ⱦ�Ϊ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾװ�ã���������ȡ�۲�Fe(OH)2�ڿ����б���������ɫ�仯��ʵ��ʱ����ʹ����м��6 mol��L��1�����ᣬ�����Լ���ѡ��

��д���пհף�
��1��B��ʢ��һ������NaOH��Һ��A��ӦԤ�ȼ������________��A�з�Ӧ�����ӷ���ʽ��________��
��2��ʵ�鿪ʼʱӦ�Ƚ�����a����Ŀ����____________________��
��3����������Fe(OH)2�IJ������̣�_______________________
��4��ʵ����ϣ���b������������һ���ֿ�������ʱB�з�����Ӧ�Ļ�ѧ����ʽΪ____��
��5��ͼ��________(��ܡ����ܡ�)�ϳ�ʱ�俴��Fe(OH)2��ɫ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com