
ΓΨΧβΡΩΓΩI. ‘Ύ“ΜΕ®ΧθΦΰœ¬Θ§Ω…ΡφΖ¥”ΠAΘΪB![]() mC±δΜ·»γΆΦΥυ ΨΓΘ“―÷ΣΉίΉχ±ξ±μ Ψ‘Ύ≤ΜΆ§Έ¬Ε»ΚΆ―Ι«Ωœ¬…ζ≥…ΈοC‘ΎΜλΚœΈο÷–ΒΡ÷ ΝΩΖ÷ ΐΘ§pΈΣΖ¥”Π‘ΎT2Έ¬Ε» ±¥οΒΫΤΫΚβΚσœρ»ίΤςΦ”―ΙΒΡ±δΜ·«ιΩωΓΘ
mC±δΜ·»γΆΦΥυ ΨΓΘ“―÷ΣΉίΉχ±ξ±μ Ψ‘Ύ≤ΜΆ§Έ¬Ε»ΚΆ―Ι«Ωœ¬…ζ≥…ΈοC‘ΎΜλΚœΈο÷–ΒΡ÷ ΝΩΖ÷ ΐΘ§pΈΣΖ¥”Π‘ΎT2Έ¬Ε» ±¥οΒΫΤΫΚβΚσœρ»ίΤςΦ”―ΙΒΡ±δΜ·«ιΩωΓΘ

Θ®1Θ©Έ¬Ε»T1________T2(ΧνΓΑ¥σ”ΎΓ±ΓΑΒ»”ΎΓ±ΜρΓΑ–Γ”ΎΓ±)ΓΘ
Θ®2Θ©’ΐΖ¥”Π «________Ζ¥”Π(ΧνΓΑΈϋ»»Γ±ΜρΓΑΖ≈»»Γ±)ΓΘ
Θ®3Θ©»γΙϊAΓΔBΓΔCΨυΈΣΤχΧεΘ§‘ρm________2(ΧνΓΑ¥σ”ΎΓ±ΓΑΒ»”ΎΓ±ΜρΓΑ–Γ”ΎΓ±)ΓΘ
Θ®4Θ©Β±Έ¬Ε»ΚΆ»ίΜΐ≤Μ±δ ±Θ§»γ‘ΎΤΫΚβΧεœΒ÷–Φ”»κ“ΜΕ®ΝΩΒΡΡ≥œΓ”–ΤχΧεΘ§‘ρΧεœΒΒΡ―Ι«Ω________(ΧνΓΑ‘ω¥σΓ±ΓΑΦθ–ΓΓ±ΜρΓΑ≤Μ±δΓ±)Θ§ΤΫΚβ_________________“ΤΕ·(ΧνΓΑœρ’ΐΖ¥”ΠΖΫœρΓ±ΓΑœρΡφΖ¥”ΠΖΫœρΓ±ΜρΓΑ≤ΜΓ±)ΓΘ
II. CO «…ζ≤ζτ ΜυΜ·―ßΤΖΒΡΜυ±Ψ‘≠ΝœΘ§ 850Γφ ±Θ§‘ΎΚψ»ίΟή±’»ίΤς÷–Ά®»κCOΚΆH2O(g)Θ§ΖΔ…ζΘΚCO(g)ΘΪH2O(g) ![]() H2(g)ΘΪCO2(g)
H2(g)ΘΪCO2(g) ![]() HΘΦ0Θ§≤βΕ®≈®Ε»Υφ ±ΦδΙΊœΒ»γœ¬±μΘΚ
HΘΦ0Θ§≤βΕ®≈®Ε»Υφ ±ΦδΙΊœΒ»γœ¬±μΘΚ
t/min | c(CO)/ molΓΛLΘ≠1 | c(H2O)/ molΓΛLΘ≠1 |
0 | 0.30 | 0.20 |
2 | \ | 0.10 |
3 | 0.18 | \ |
4 | \ | 0.08 |
ΜΊ¥πœ¬Ν–Έ Χβ
Θ®1Θ©t=3min ±Θ§Π‘(’ΐ)____________Π‘(Ρφ)Θ®―ΓΧνΘΚΓΑΘΨΓ±Θ§ΓΑΘΦΓ±Θ§ΓΑΘΫΓ±Θ©ΓΘ
Θ®2Θ©0ΓΪ2minΘ§COΒΡΤΫΨυΖ¥”ΠΥΌ¬ ΈΣ ____________ΓΘ
Θ®3Θ©…œ ωΖ¥”Π÷–ΒΡCOΤΫΚβΉΣΜ·¬ ΈΣ____________ΓΘ
Θ®4Θ©‘Ύ“ΜΕ®ΧθΦΰœ¬Θ§ΖΔ…ζœ¬Ν–Ζ¥”ΠΘΚCO(g)ΘΪH2O(g) ![]() H2(g)ΘΪCO2(g)Θ§ΤδΡφΖ¥”ΠΒΡΥΌ¬ Υφ ±Φδ±δΜ·«ζœΏ»γΆΦΥυ ΨΘ§‘ρt1 ±ΗΡ±δΒΡΡ≥÷÷Ζ¥”ΠΧθΦΰΩ…Ρή «_____Θ®Χν–ρΚ≈Θ©ΓΘ
H2(g)ΘΪCO2(g)Θ§ΤδΡφΖ¥”ΠΒΡΥΌ¬ Υφ ±Φδ±δΜ·«ζœΏ»γΆΦΥυ ΨΘ§‘ρt1 ±ΗΡ±δΒΡΡ≥÷÷Ζ¥”ΠΧθΦΰΩ…Ρή «_____Θ®Χν–ρΚ≈Θ©ΓΘ

aΘ°‘ω¥σCO≈®Ε»
bΘ°Φ”―Ι
cΘ°Κψ»ί ±≥δ»κκ≤Τχ
dΘ° Ι”Ο¥ΏΜ·ΦΝ
ΓΨ¥πΑΗΓΩ¥σ”Ύ Ζ≈»» ¥σ”Ύ ‘ω¥σ ≤Μ ΘΫ ![]() 40% bd
40% bd
ΓΨΫβΈωΓΩ
I.Θ®1)Έ¬Ε»‘ΫΗΏΖ¥”ΠΥΌ¬ ‘ΫΩλΘ§ΒΫ¥οΤΫΚβΒΡ ±Φδ‘ΫΕΧΘΜ
(2)”…ΆΦΩ…÷ΣΈ¬Ε»T1ΘΨT2Θ§Έ¬Ε»‘ΫΗΏC%‘Ϋ–ΓΘ§Ι …ΐΗΏΈ¬Ε»ΤΫΚβœρΡφΖ¥”Π“ΤΕ·ΘΜ
(3)”…ΆΦΩ…÷ΣΘ§T2Έ¬Ε» ±¥οΒΫΤΫΚβΚσœρ»ίΤςΦ”―ΙΘ§C%Φθ–ΓΘ§Ι ‘ω¥σ―Ι«ΩΤΫΚβœρΡφΖ¥”Π“ΤΕ·ΘΜ
(4)Β±Έ¬Ε»ΚΆ»ίΜΐ≤Μ±δ ±Θ§‘ΎΤΫΚβΧεœΒ÷–Φ”»κ“ΜΕ®ΝΩΒΡΡ≥œΓ”–ΤχΧεΘ§ΧεœΒ―Ι«Ω‘ω¥σΘ§ΒΪΖ¥”ΠΜλΚœΈοΒΡ≈®Ε»≤Μ±δΘ§ΤΫΚβ≤Μ“ΤΕ·ΘΜ
II.(1)(2)(3)άϊ”Ο»ΐΕΈ ΫΫχ––ΦΤΥψΘ§Ω…“‘ΒΟ≥ω3minΡ©c(H2O)Θ§ΥΒΟς¥Υ ±“―Ψ≠¥οΒΫΜ·―ßΤΫΚβΘ§άϊ”ΟΜ·―ßΖ¥”ΠΥΌ¬ ΒΡΦΤΥψΙΪ Ϋ≤ΜΡ―Υψ≥ωCOΒΡΖ¥”ΠΥΌ¬ ΚΆ![]() Θ§Φ¥Ω…“‘«σ≥ωCOΒΡΉΣΜ·¬ Θ§ΉνΚσ(4)ΫαΚœΆΦ Ψ–≈œΔΚΆά’œΡΧΊΝ–‘≠άμΫχ––Ϋβ¥πΓΘ
Θ§Φ¥Ω…“‘«σ≥ωCOΒΡΉΣΜ·¬ Θ§ΉνΚσ(4)ΫαΚœΆΦ Ψ–≈œΔΚΆά’œΡΧΊΝ–‘≠άμΫχ––Ϋβ¥πΓΘ
I.(1)”…ΆΦΩ…÷ΣΘ§Έ¬Ε»ΈΣT1œ»ΒΫ¥οΤΫΚβΘ§Έ¬Ε»‘ΫΗΏΖ¥”ΠΥΌ¬ ‘ΫΩλΘ§ΒΫ¥οΤΫΚβΒΡ ±Φδ‘ΫΕΧΘ§Ι T1ΘΨT2Θ§Ι ¥πΑΗΈΣΘΚ¥σ”ΎΘΜ
(2)”…ΆΦΩ…÷ΣΈ¬Ε»T1ΘΨT2Θ§Έ¬Ε»‘ΫΗΏC%‘Ϋ–ΓΘ§Ι …ΐΗΏΈ¬Ε»ΤΫΚβœρΡφΖ¥”Π“ΤΕ·Θ§Ι ’ΐΖ¥”ΠΈΣΖ≈»»Ζ¥”ΠΘ§Ι ¥πΑΗΈΣΘΚΖ≈»»ΘΜ
(3)”…ΆΦΩ…÷ΣΘ§T2Έ¬Ε» ±¥οΒΫΤΫΚβΚσœρ»ίΤςΦ”―ΙΘ§C%Φθ–ΓΘ§Ι ‘ω¥σ―Ι«ΩΤΫΚβœρΡφΖ¥”Π“ΤΕ·Θ§‘ω¥σ―Ι«ΩΤΫΚβœρΤχΧεΈο÷ ΒΡΝΩΦθ–ΓΒΡΖΫœρ“ΤΕ·Θ§ΥΒΟςΗΟΖ¥”ΠΡφΖ¥”ΠΖΫœρ «ΤχΧεΈο÷ ΒΡΝΩΦθ–ΓΒΡΖΫœρΘ§Ι mΘΨ2Θ§¥πΑΗΈΣΘΚ¥σ”ΎΘΜ
(4)Β±Έ¬Ε»ΚΆ»ίΜΐ≤Μ±δ ±Θ§‘ΎΤΫΚβΧεœΒ÷–Φ”»κ“ΜΕ®ΝΩΒΡΡ≥œΓ”–ΤχΧεΘ§“ρΤχΧεΒΡΈο÷ ΒΡΝΩ‘ω¥σΘ§ΧεœΒ―Ι«Ω‘ω¥σΘ§ΒΪΖ¥”ΠΜλΚœΈοΒΡ≈®Ε»≤Μ±δΘ§’ΐΓΔΡφΖ¥”ΠΥΌ¬ ≤Μ±δΘ§ΤΫΚβ≤Μ“ΤΕ·Θ§Ι ¥πΑΗΈΣΘΚ‘ω¥σΘ§≤ΜΘΜ
II. (1)ΗυΨί»ΐΕΈ ΫΦΤΥψΘ§ΡήΙΜΚήΩλΦΤΥψ≥ω3minΡ©Θ§c(H2O)=0.08 molΓΛLΘ≠1”κ4minΡ©“Μ―υΘ§ΥΒΟςt=3min ±Ζ¥”Π“―Ψ≠¥οΒΫΜ·―ßΤΫΚβΘ§Π‘(’ΐ) = Π‘(Ρφ)Θ§Ι ¥πΑΗΈΣΘΚΘΫΘΜ
(2)ΗυΨίΖ¥”ΠΖΫ≥Χ ΫΩ…÷ΣΘ§0ΓΪ2min COΒΡΈο÷ ΒΡΝΩ≈®Ε»±δΜ·ΝΥ0.1 molΓΛLΘ≠1Θ§Ι ΤΫΨυΖ¥”ΠΥΌ¬ ΈΣ![]() Θ§Ι ¥πΑΗΈΣΘΚ
Θ§Ι ¥πΑΗΈΣΘΚ![]() ΘΜ
ΘΜ
(3)¥”±μ÷– ΐΨίΩ…÷ΣΘ§¥οΒΫΤΫΚβ ±Θ§COΒΡΤΫΚβ≈®Ε»ΈΣ0.18 molΓΛLΘ≠1Θ§Ι …œ ωΖ¥”Π÷–ΒΡCOΤΫΚβΉΣΜ·¬ ΈΣΘΚ![]() Θ§Ι ¥πΑΗΈΣΘΚ40%
Θ§Ι ¥πΑΗΈΣΘΚ40%
(4)¥”ΆΦ÷–Ω…÷ΣΘ§ΗΡ±δΧθΦΰΚσΘ§ΡφΖ¥”ΠΥΌ¬ ΆΜ»Μ‘ω¥σΘ§ΒΪ «‘ω¥σΚσΤΫΚβ≤Μ“ΤΕ·ΓΘ
aΘ°‘ω¥σCO≈®Ε»Θ§’ΐΖ¥”ΠΥΌ¬ ΆΜ»Μ‘ω¥σΘ§ΡφΖ¥”ΠΥΌ¬ ‘ρ «¬ΐ¬ΐ‘ω¥σΚσ≤Μ±δΘ§≤ΜΖϊΚœΆΦ Ψ–≈œΔΘ§a≤ΜΚœΧβ“βΘΜ
bΘ°”…”ΎCO(g)ΘΪH2O(g)![]() H2(g)ΘΪCO2(g)Ζ¥”Π«ΑΚσΤχΧεΒΡΈο÷ ΒΡΝΩ±Θ≥÷≤Μ±δΘ§Ι Φ”―Ι ΙΒΟ’ΐΓΔΡφΖ¥”ΠΥΌ¬ Ά§Β»ΖυΕ»ΒΊΆΜ»Μ‘ω¥σΘ§ΤΫΚβ≤Μ“ΤΕ·Θ§ΖϊΚœΆΦ Ψ–≈œΔΘ§bΖϊΚœΧβ“βΘΜ
H2(g)ΘΪCO2(g)Ζ¥”Π«ΑΚσΤχΧεΒΡΈο÷ ΒΡΝΩ±Θ≥÷≤Μ±δΘ§Ι Φ”―Ι ΙΒΟ’ΐΓΔΡφΖ¥”ΠΥΌ¬ Ά§Β»ΖυΕ»ΒΊΆΜ»Μ‘ω¥σΘ§ΤΫΚβ≤Μ“ΤΕ·Θ§ΖϊΚœΆΦ Ψ–≈œΔΘ§bΖϊΚœΧβ“βΘΜ
cΘ°Κψ»ί ±≥δ»κκ≤ΤχΘ§ΧεœΒΒΡ―Ι«Ω‘ω¥σΘ§ΒΪ «Ζ¥”ΠΈο”κ…ζ≥…ΈοΒΡ≈®Ε»ΨυΈ¥ΗΡ±δΘ§Ι ’ΐΓΔΡφΖ¥”ΠΥΌ¬ Ψυ≤Μ±δΘ§≤ΜΖϊΚœΆΦ Ψ–≈œΔΘ§c≤ΜΚœΧβ“βΘΜ
dΘ° Ι”Ο¥ΏΜ·ΦΝΡήΙΜΆ§Β»ΖυΕ»ΒΊΆΜ»Μ‘ω¥σ’ΐΓΔΡφΖ¥”ΠΥΌ¬ Θ§ΤΫΚβ≤Μ“ΤΕ·Θ§ΖϊΚœΆΦ Ψ–≈œΔΘ§dΖϊΚœΧβ“βΘΜ
Ι ¥πΑΗΈΣΘΚbdΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ‘ΎΗχΕ®ΧθΦΰœ¬Θ§œ¬Ν–―ΓœνΥυ ΨΒΡΈο÷ ΦδΉΣΜ·ΨυΡή Βœ÷ΒΡ «
A.H2SiO3![]() SiO2
SiO2 ![]() SiCl4
SiCl4
B.Cu![]() Cu(NO3)2(aq)
Cu(NO3)2(aq) ![]() Cu(NO3)2(s)
Cu(NO3)2(s)
C.ClCH2Θ≠CH2Cl![]() HOCH2Θ≠CH2OH
HOCH2Θ≠CH2OH ![]() HOOCΘ≠COOH
HOOCΘ≠COOH
D.Al![]() Al2O3
Al2O3![]() NaAlO2(aq)
NaAlO2(aq)
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΚ§¬»œϊΕΨΦΝΩ…”––ß…±Οπ–¬ΙΎ≤ΓΕΨΘ®2019-nCoVΘ©ΓΘΡ≥―ßœΑ–ΓΉιΗυΨί–η“Σ”ϊ÷Τ±Η≈®Ε»≤Μ–Γ”Ύ0.8mol/LΒΡ¥Έ¬»Υα»ή“ΚΓΘΉΑ÷ΟΦϊœ¬ΆΦΓΘ
Ή Νœ1ΘΚ≥ΘΈ¬≥Θ―Ιœ¬Θ§Cl2OΈΣΉΊΜΤ…ΪΤχΧεΘ§Ζ–ΒψΈΣ3.8ΓφΘ§42Γφ“‘…œΜαΖ÷Ϋβ…ζ≥…Cl2ΚΆO2Θ§Cl2O“Ή»ή”ΎΥ°≤Δ”κΥ°ΝΔΦ¥Ζ¥”Π…ζ≥…HClOΓΘ
Ή Νœ2ΘΚΫΪ¬»ΤχΚΆΩ’Τχ(≤Μ≤Έ”κΖ¥”Π)Α¥ΧεΜΐ±» 1ΓΟ3ΜλΚœΆ®»κΚ§Υ°8%ΒΡΧΦΥαΡΤ÷–÷ΤCl2OΘ§≤Δ”ΟΥ°Έϋ ’Cl2O(≤ΜΚ§Cl2)÷ΤΒΟ¥Έ¬»Υα»ή“ΚΓΘ
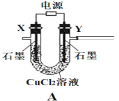

Θ®1Θ©ΒγΦΪY”ΠΗΟΝ§Ϋ”Βγ‘¥ΒΡ___Θ®ΧνΓΑ’ΐΦΪΓ±ΜρΓΑΗΚΦΪΓ±Θ©,ΉΑ÷ΟCΡΎ”Π ΔΖ≈_________Θ§ΗςΉΑ÷ΟΒΡΝ§Ϋ”Υ≥–ρΈΣAΓΣ________________________________
Θ®2Θ©Ζ¥”ΠΙΐ≥Χ÷–Θ§ΉΑ÷ΟB–ηΖ≈‘ΎάδΥ°÷–Θ§ΤδΡΩΒΡ «_____________________________ΓΘ“―÷ΣΉΑ÷ΟB≤ζΈοΚ§”–“Μ÷÷Υα Ϋ―ΈΘ§‘ρΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ___________________ΓΘ
Θ®3Θ© Β―ι÷–ΩΊ÷Τ¬»Τχ”κΩ’ΤχΧεΜΐ±»ΒΡΖΫΖ® «_______________________________
Θ®4Θ©ΉΑ÷ΟE÷– Ι”ΟΉΊ…ΪΤΫΒΉ…’ΤΩΒΡ‘≠“ρ «Θ®”ΟΜ·―ßΖΫ≥Χ Ϋ±μ ΨΘ©________________
Θ®5Θ©¥ΥΖΫΖ®œύΕ‘”Ύ”Ο¬»Τχ÷±Ϋ”»ή”ΎΥ°÷Τ±Η¥Έ¬»Υα»ή“ΚΒΡ”≈Βψ «Θ®¥π≥ω“ΜΧθΦ¥Ω…Θ©_____ΓΘ
Θ®6Θ©»τΉΑ÷ΟB÷–…ζ≥…ΒΡCl2OΤχΧε”–20%÷ΆΝτ‘ΎE«ΑΗςΉΑ÷Ο÷–Θ§Τδ”ύΨυ»ή”ΎΉΑ÷ΟEΒΡΥ°÷–Θ§ΉΑ÷ΟEΥυΒΟ500mL¥Έ¬»Υα»ή“Κ≈®Ε»ΈΣ0.8mol/L,‘ρ÷Ν…Ό–η“ΣΚ§Υ°8%ΒΡΧΦΥαΡΤΒΡ÷ ΝΩΈΣ___gΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ(1)ΫϋΡξά¥Θ§ΥφΉ≈ΨέθΞΙΛ“ΒΒΡΩλΥΌΖΔ’ΙΘ§¬»ΤχΒΡ–η«σΝΩΚΆ¬»Μ·«βΒΡ≤ζ≥ωΝΩ“≤Υφ÷°―ΗΥΌ‘ω≥ΛΓΘ“ρ¥ΥΘ§ΫΪ¬»Μ·«βΉΣΜ·ΈΣ¬»ΤχΒΡΦΦ θ≥…ΈΣΩΤ―ß―–ΨΩΒΡ»»ΒψΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚDeacon÷±Ϋ”―θΜ·Ζ®Ω…Α¥œ¬Ν–¥ΏΜ·Ιΐ≥ΧΫχ––ΘΚ
CuCl2(s)=CuCl(s)+![]() Cl2(g) ΠΛH1=83 kJΓΛmol 1
Cl2(g) ΠΛH1=83 kJΓΛmol 1
CuCl(s)+![]() O2(g)=CuO(s)+
O2(g)=CuO(s)+![]() Cl2(g) ΠΛH2= 20 kJΓΛmol 1
Cl2(g) ΠΛH2= 20 kJΓΛmol 1
CuO(s)+2HCl(g)=CuCl2(s)+H2O(g) ΠΛH3= 121 kJΓΛmol 1
‘ρ4HCl(g)+O2(g)=2Cl2(g)+2H2O(g)ΒΡΠΛH=_________ kJΓΛmol 1ΓΘΤδ÷–O2ΒΡΒγΉ” ΫΈΣ__________
(2)ΙηΖέ”κHCl‘Ύ300Γφ ±Ζ¥”Π…ζ≥…1mol SiHCl3ΤχΧεΚΆH2 Θ§Ζ≈≥ω225KJ»»ΝΩΘ§ΗΟΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣ________________________ΓΘSiHCl3÷–Κ§”–ΒΡΜ·―ßΦϋάύ–ΆΈΣ__________
(3)ΫΪSiCl4«βΜ·ΈΣSiHCl3”–»ΐ÷÷ΖΫΖ®Θ§Ε‘”ΠΒΡΖ¥”Π“ά¥ΈΈΣΘΚ
ΔΌSiCl4(g)+H2(g)![]() SiHCl3(g)+HCl(g) ΠΛH1ΘΨ0
SiHCl3(g)+HCl(g) ΠΛH1ΘΨ0
ΔΎ3SiCl4(g)+2H2(g)+Si(s)![]() 4SiHCl3(g) ΠΛH2ΘΦ0
4SiHCl3(g) ΠΛH2ΘΦ0
Δέ2SiCl4(g)+H2(g)+Si(s)+HCl(g)![]() 3SiHCl3(g) ΠΛH3
3SiHCl3(g) ΠΛH3
‘ρΖ¥”ΠΔέΒΡΠΛH3______(”ΟΠΛH1Θ§ΠΛH2±μ Ψ)ΓΘ
(4)Εΰ―θΜ·¬» «ΡΩ«ΑΙζΦ …œΙΪ»œΒΡΒΎΥΡ¥ζΗΏ–ßΓΔΈόΕΨΒΡΙψΤΉœϊΕΨΦΝ,ΥϋΩ…”…KClO3‘ΎH2SO4¥φ‘Ύœ¬”κNa2SO3Ζ¥”Π÷ΤΒΟ.«κ–¥≥ωΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ________________________
(5)¬»Μ·οß≥Θ”ΟΉςΚΗΫ”Θ°»γΘΚ‘ΎΚΗΫ”Ά≠Τς ±”Ο¬»Μ·οß≥ΐ»ΞΆ≠Τς±μΟφΒΡ―θΜ·Ά≠“‘±ψΚΗΫ”Θ§ΤδΖ¥”ΠΈΣΘΚ_______CuO+______NH4Cl![]() ______Cu+______CuCl2+______N2Γϋ+______H2O
______Cu+______CuCl2+______N2Γϋ+______H2O
ΔΌ≈δΤΫ¥Υ―θΜ·ΜΙ‘≠Ζ¥”ΠΖΫ≥Χ Ϋ___________________________
ΔΎ¥ΥΖ¥”Π÷–»τ≤ζ…ζ0.2molΒΡΤχΧεΘ§‘ρ”–__________molΒΡΒγΉ”ΉΣ“ΤΘ°
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ…ηNA±μ ΨΑΔΖϋΦ”Β¬¬ό≥Θ ΐΒΡ÷ΒΓΘœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «
A. 0.5mol–έΜΤ(As4S4)Θ§ΫαΙΙ»γ”“ΆΦΘ§Κ§”–NAΗωS-SΦϋ![]()
B. ΫΪ1molNH4NO3»ή”Ύ ΝΩœΓΑ±Υ°÷–Θ§ΥυΒΟ»ή“Κ≥ ÷––‘Θ§‘ρ»ή“Κ÷–NH4+ΒΡ ΐΡΩΈΣNA
C. ±ξΉΦΉ¥Ωωœ¬Θ§33.6LΕ଻ֹΆι÷–Κ§”–¬»‘≠Ή”ΒΡ ΐΡΩΈΣ3NA
D. ΗΏΈ¬œ¬Θ§16.8gFe”κΉψΝΩΥ°’τΤχΆξ»ΪΖ¥”ΠΘ§ΉΣ“ΤΒΡΒγΉ” ΐΈΣ0.6NA
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΒΣ(N)ΓΔœθ(P)ΓΔ…ι(As)»¾VAΉε‘ΣΥΊΒΡΜ·ΚœΈο‘Ύ―–ΨΩΚΆ…ζ≤ζ÷–”–÷Ί“Σ”ΟΆΨΘ§«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©ΜυΧ§…ι‘≠Ή”ΒΡΦέΒγΉ”≈≈≤ΦΆΦΈΣ________________Θ§ΒΎ»ΐ÷ήΤΎ‘ΣΥΊΒΡ‘≠Ή”÷–Θ§ΒΎ“ΜΒγάκΡή¥σ”ΎΝΉ‘≠Ή”ΒΡ”–________________÷÷ΓΘ
Θ®2Θ©ΒΣΉε‘ΣΥΊ¬»Μ·ΈοRH3(NH3ΓΔPH3ΓΔAsH3)ΒΡΡ≥÷÷–‘÷ ΥφRΒΡΚΥΒγΚ… ΐΒΡ±δΜ·«ς Τ»γΆΦΥυ ΨΘ§‘ρY÷αΩ…±μ ΨΒΡ«βΜ·Έο(RH3)–‘÷ Ω…Ρή”–________________(Χν–ρΚ≈)ΓΘ
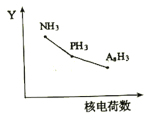
A.Έ»Ε®–‘B.Ζ–Βψ ±C.RΘ≠HΦϋ÷°ΦδΒΡΦϋΫ«D.Ζ÷Ή”ΦδΉς”ΟΝΠE.ΜΙ‘≠–‘
Θ®3Θ©Ψ≠≤βΕ®ΖΔœ÷Θ§Ρ≥÷÷N2O5ΙΧΧε”…NO2ΘΪΚΆNO3Θ≠ΝΫ÷÷άκΉ”Ήι≥…Θ§―τάκΉ”÷–N‘≠Ή”ΒΡ‘”Μ·ΖΫ Ϋ «________________‘”Μ·Θ§“θάκΉ”ΒΡΩ’ΦδΙΙ–ΆΈΣ________________ΓΘ
Θ®4Θ©““ΕΰΑΖ(H2NΘ≠CH2Θ≠CH2Θ≠NH2)”κCuCl2»ή“ΚΩ…–Έ≥…≈δάκΉ”(ΫαΙΙ»γΆΦ)ΓΘ
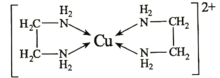
Cu2ΘΪΒΡ≈δΈΜ ΐΈΣ________________Θ§1molΗΟ≈δάκΉ”÷–Κ§Π“ΦϋΈΣ________________molΘ§““ΕΰΑΖΚΆ»ΐΦΉΑΖ[N(CH3)3]Ψυ τ”ΎΑΖΘ§ΒΪ““ΕΰΑΖ±»»ΐΦΉΑΖΒΡΖ–ΒψΗΏΒΟΕύΘ§Τδ‘≠“ρ «________________ΓΘ
Θ®5Θ©Ά®≥Θ»œΈΣCu3N «άκΉ”ΨßΧεΘ§ΤδΨßΗώΡήΩ…Ά®ΙΐΆΦ1ΒΡBornΘ≠Haber―≠ΜΖΦΤΥψΒΟΒΫΓΘΆ®ΙΐΆΦ1÷– ΐΨί________________(ΧνΓΑΡήΓ±ΜρΓΑ≤ΜΡήΓ±)ΦΤΥψ≥ωCu‘≠Ή”ΒΡΒΎ“ΜΒγάκΡήΘ§Cu3NΨßΗώΡήΈΣ________________kJΓΛmolΘ≠1ΓΘ
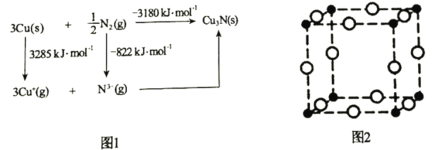
Θ®6Θ©Cu3NΨßΑϊ»γΆΦ2Υυ ΨΘ§CuΘΪΒΡΑκΨΕΈΣapmΘ§N3Θ≠ΒΡΑκΨΕΈΣbpmΘ§Cu3NΨßΑϊΒΡΟήΕ»ΈΣ________________gΓΛcmΘ≠3(Ν–≥ωΦΤΥψ ΫΦ¥Ω…Θ§ΑΔΖϋΦ”Β¬¬ό≥Θ ΐΒΡ÷Β”ΟNA±μ Ψ)ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–Ζ¥”Π÷– τ”ΎΦ”≥…Ζ¥”ΠΒΡ «(ΓΓΓΓ)
A. CH4ΘΪCl2![]() CH3ClΘΪHCl
CH3ClΘΪHCl
B. CH2===CH2ΘΪHCl®DΓζCH3CH2Cl
C. CH3CH2OHΘΪHBr![]() CH3CH2BrΘΪH2O
CH3CH2BrΘΪH2O
D. 2CH3CH3ΘΪ7O2![]() 4CO2ΘΪ6H2O
4CO2ΘΪ6H2O
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ“ΜΕ®Έ¬Ε»œ¬‘Ύ“ΜΗω2 LΒΡΚψ»ίΟή±’»ίΤς÷–ΖΔ…ζΖ¥”Π4A(s)+3B(g)2C(g)+D(g)Θ§Ψ≠2 min¥οΤΫΚβΉ¥Χ§Θ§¥Υ ±BΖ¥”ΠœϊΚΡΝΥ0.9 molΘ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «
A. ΤΫΚβ ±Θ§v(A)ΓΟv(B)ΓΟv(C)ΓΟv(D) =4ΓΟ3ΓΟ2ΓΟ1
B. ΜλΚœΤχΒΡΤΫΨυœύΕ‘Ζ÷Ή”÷ ΝΩΩ…ΉςΈΣΤΫΚβ±ξ÷Ψ
C. ≥δ»κΕη–‘ΤχΧε Ι―Ι«Ω‘ω¥σΩ…Φ”ΩλΖ¥”ΠΥΌ¬
D. CΒΡΤΫΨυΖ¥”ΠΥΌ¬ ΈΣ0.5 mol/(LΓΛmin)
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ2019ΡξœΟΟ≈¥σ―ßΆθ“ΑΫΧ ΎΩΈΧβΉι‘ΎΚœ≥…Τχ(÷ς“Σ≥…Ζ÷COΚΆH2)¥ΏΜ·ΉΣΜ·÷Τ““¥ΦΖΫΟφ Β ©Ω…ΩΊΫ”ΝΠ¥ΏΜ·Θ§Α¥’’:Κœ≥…ΤχΓζΦΉ¥ΦΓζ““ΥαΓζ““¥ΦΒΡΖΫ ΫΘ§≥…ΙΠ Βœ÷““¥ΦΒΡΗΏ―Γ‘ώ–‘Κœ≥…ΓΘ
Θ®1Θ©“―÷ΣΘΚΔΌH2(g)+![]() O2(g)=H2O(g) ΓςH1=-285.8KJ/molΘΜΔΎCO(g)+
O2(g)=H2O(g) ΓςH1=-285.8KJ/molΘΜΔΎCO(g)+![]() O2(g)=CO2(g) ΓςH2=-283KJ/molΘΜΔέCH3OH(g)+
O2(g)=CO2(g) ΓςH2=-283KJ/molΘΜΔέCH3OH(g)+![]() O2(g)= CO2(g)+2 H2O(l) ΓςH3=-764.6 KJ/molΘΜ
O2(g)= CO2(g)+2 H2O(l) ΓςH3=-764.6 KJ/molΘΜ
‘ρΙΛ“Β…œάϊ”ΟΚœ≥…Τχ÷Τ±ΗΦΉ¥ΦΒΡΩ…ΡφΖ¥”Π»»Μ·―ßΖΫ≥Χ ΫΈΣ______________________ΘΜ
Θ®2Θ©ΚψΈ¬Κψ»ίΧθΦΰœ¬Θ§œ¬Ν–ΡήΥΒΟςΚœ≥…Τχ÷ΤΦΉ¥ΦΒΡΖ¥”Π“―¥οΤΫΚβΉ¥Χ§ΒΡ «_______ΘΜ
AΘ°ΒΞΈΜ ±ΦδΡΎ…ζ≥…n mol COΒΡΆ§ ±…ζ≥…2n mol H2
BΘ° v(H2)’ΐ= 2v(CH3OH)Ρφ
CΘ°»ίΤςΡΎΤχΧεΒΡΟήΕ»±Θ≥÷≤Μ±δ
DΘ°»ίΤς÷–ΤχΧεΒΡΤΫΨυΡΠΕϊ÷ ΝΩ±Θ≥÷≤Μ±δ
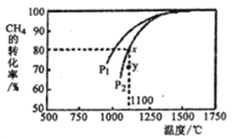
Θ®3Θ©άϊ”ΟΧλ»ΜΤχ÷Τ»ΓΚœ≥…ΤχΒΡ‘≠άμΈΣ: CO2(g)+CH4(g)= 2CO(g)+ 2H2(g)Θ§‘ΎΟή±’»ίΤς÷–Ά®»κΈο÷ ΒΡΝΩ≈®Ε»ΨυΈΣ0.1 molL-1ΒΡCH4”κCO2Θ§‘Ύ“ΜΕ®ΧθΦΰœ¬ΖΔ…ζΖ¥”ΠΘ§≤βΒΟCH4ΒΡΤΫΚβΉΣΜ·¬ ”κΈ¬Ε»ΦΑ―Ι«ΩΒΡΙΊœΒ»γœ¬ΆΦΥυ ΨΘ§‘ρ―Ι«ΩP1______P2 (ΧνΓΑ¥σ”ΎΓ±ΜρΓΑ–Γ”ΎΓ±)ΘΜ―Ι«ΩΈΣP2 ±Θ§‘ΎYΒψΘΚv(’ΐ)_____v(Ρφ) (ΧνΓΑ¥σ”ΎΓ±ΓΔΓΑ–Γ”ΎΓ±ΜρΓΑΒ»”ΎΓ±)Θ§«σYΒψΕ‘”ΠΈ¬Ε»œ¬ΒΡΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐK=________ (ΦΤΥψΫαΙϊ±ΘΝτΝΫΈΜ”––ß ΐΉ÷)ΓΘ
Θ®4Θ©“‘Εΰ―θΜ·ν―±μΟφΗ≤Η«CuAlO4ΈΣ¥ΏΜ·ΦΝΘ§Ω…“‘ΫΪCH4ΚΆCO2÷±Ϋ”ΉΣΜ·≥…““ΥαΓΘ
ΔΌ‘Ύ≤ΜΆ§Έ¬Ε»œ¬¥ΏΜ·ΦΝΒΡ¥ΏΜ·–߬ ”κ““ΥαΒΡ…ζ≥…ΥΌ¬ »γœ¬ΆΦΥυ ΨΓΘ250-300ΓψC ±Θ§Έ¬Ε»…ΐΗΏΟφ““ΥαΒΡ…ζ≥…ΥΌ¬ ΫΒΒΆΒΡ‘≠“ρ «____ΘΜ
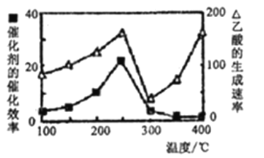
ΔΎΈΣΝΥΧαΗΏΗΟΖ¥”Π÷–CH4ΒΡΉΣΜ·¬ Θ§Ω…“‘≤…»ΓΒΡ¥κ © «_____________________ΘΜ
Θ®5Θ©“ΜΕ®ΧθΦΰœ¬Θ§ΦΉ¥Φ”κ“Μ―θΜ·ΧΦΖ¥”ΠΩ…“‘Κœ≥…““ΥαΓΘ≥ΘΈ¬ΧθΦΰœ¬Θ§ΫΪa mol/LΒΡCH3COOH»ή“Κ”κb mol/ Ba(OH)2»ή“ΚΒ»ΧεΜΐΜλΚœΘ§Ζ¥”ΠΤΫΚβ ±Θ§2c(Ba2+)=c(CH3COO-)Θ§”ΟΚ§aΚΆbΒΡ¥ζ ΐ Ϋ±μ ΨΘ§ΗΟΜλΚœ»ή“Κ÷–¥ΉΥαΒΡΒγάκ≥Θ ΐΈΣ________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com