
����Ŀ��ʵ�������ܶ�Ϊ1.84 g/cm3�����ʵ���������Ϊ98 %�����ᣬ����250 mL���ʵ���Ũ��Ϊ0.46 mol/L�����ᡣ
(1)98 %��Ũ��������ʵ���Ũ��Ϊ_________��
(2)�������м��ֹ�����Ͳ��Ӧѡ��______������ţ���
��5 mL��Ͳ ��10 mL��Ͳ ��50 mL��Ͳ ��100 mL��Ͳ
(3)ʵ����Ҫ���²��裺
�ٶ��ݢ���ȡ��ҡ�Ȣ�ϴ�Ӣ�ת�Ƣ���ȴ�����װƿ��ϡ�ͽ���˳��Ϊ_______��
(4)���в���ʹ������Һ�����ʵ���Ũ��ƫ�ߵ���__________��
A��������ƿ��ת����Һʱ������Һ�彦��
B��δϴ��ϡ��ŨH2SO4��С�ձ�
C������ʱ���ӿ̶���
D��ϴ������ƿδ���T����������Һ
E�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶���
���𰸡�18.4 mol/L �� �ߢڢ�ޢݢܢ٢ۢ� C
��������
(1)����c=![]() ������Ũ��������ʵ���Ũ�ȣ�
������Ũ��������ʵ���Ũ�ȣ�
(2)������Һϡ�������������ʵ����ʵ������������ҪŨ��������������Ũ�������ѡ����ʵ���Ͳ��
(3)����һ�����ʵ���Ũ����Һһ�㲽�裺���㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��ݴ�����
(4)�������������ʵ����ʵ���n����Һ���V��Ӱ�죬����c=![]() ���з���������ʹnƫ����ʹVƫС�IJ���������ʹ��ҺŨ��ƫ�ߣ���֮����ҺŨ�Ⱦ�ƫ����
���з���������ʹnƫ����ʹVƫС�IJ���������ʹ��ҺŨ��ƫ�ߣ���֮����ҺŨ�Ⱦ�ƫ����
(1)�ܶ�Ϊ1.84g/cm3�����ʵ���������Ϊ98%�����ᣬ���ʵ���Ũ��c=![]() =18.4mol/L��
=18.4mol/L��
(2)����250mL���ʵ���Ũ��Ϊ0.46mol/L�����ᣬ����ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ����������ã�18.4mol/L��V=0.46mol/L��250mL�����V=6.3mL������Ӧѡ��10mL��Ͳ����Ϊ����
(3)����һ�����ʵ���Ũ����Һһ�㲽�裺���㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ�������ȷ��˳��Ϊ���ߢڢ�ޢݢܢ٢ۢ�
(4)A��������ƿ��ת����Һʱ������Һ�彦�������²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ�A������
B��δϴ��ϡ��ŨH2SO4��С�ձ������²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ�B������
C������ʱ���ӿ̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ�C��ȷ��
D��ϴ������ƿδ���T����������Һ�������ʵ����ʵ�������Һ������������Ӱ�죬��ҺŨ�Ȳ��䣬D������
E�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ����ټ�ˮ���̶��ߣ��ᵼ����Һ���ƫ����ҺŨ��ƫ�ͣ�E������
�ʺ���ѡ����C��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��W��X��Y��Z��Q��F������ԭ���������������ǰ������Ԫ�أ�����W�����������������ڲ��������3����X�Ƕ�������ԭ�Ӱ뾶����Ԫ�أ�X��Y��ZΪͬ����Ԫ�أ�Yԭ�ӵĺ��������������������������3����Zԭ���������1��δ�ɶԵ��ӣ�Qλ��Y�IJ�ͬ���ڣ���������ɵ�������ͬ���������ģ�FԪ�ػ�̬ԭ���������1�����ӡ���ش��������⣺
��1��W��X��Y��Z����Ԫ���е�һ��������С����__________����Ԫ�ط��ţ���Qԭ�ӵļ۵����Ų�ʽΪ________��
��2��ZԪ�������ڱ��е�λ��Ϊ___________________��
��3��WԪ��������ͬ�������壬 W��X�γɵĻ������к��зǼ��Թ��ۼ�������P��д��P��W������⻯�ﷴӦ�����ӷ���ʽ__________________
��4����ͼ��WԪ�ص����������Ų�ͼ����Υ���˵����Ų���ʲôԭ��____________ ��
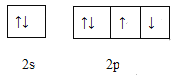
��5��Y���ʺ�Z���ʷ�Ӧ������ԭ�Ӹ�����Ϊ1��5�Ļ�����M��1:3�Ļ�����N����M�м�̬��ͬ��Y�ĺ�����������幹��Ϊ_____________��
��6��д��������N�ĵ���ʽ��__________________________
��7��ij��������F(��)(���ʾ���ϼ�Ϊ��1)����γ�ͼ��ʾ�����ӣ���������̼ԭ�ӵ��ӻ���ʽ��____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ӽ���Ĥ�������ӽ���Ĥ��ʯī�缫����ͼ��ʾ�ĵ��ۡ������ȼҵ�е����ӽ���Ĥ����ԭ�����ɵ��Na2SO4��Һ����NaOH��Һ��H2SO4��Һ������˵���в���ȷ����

A. ������ӦʽΪ4OH--4e-=2H2O+O2��
B. ��A�ڳ�������������Һ
C. b�������ӽ���Ĥ������Na+ͨ��
D. Na2SO4��Һ��E�ڼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
��1��д������װ��������ͬ������������Ӧ�Ļ�ѧ����ʽ: �� ��
д��BʢAgNO3��Һ���Թ�����������Ӧ�Ļ�ѧ����ʽ�� ��
��2��װ��A��C�������˳��������ܣ��������� ��
��3���ڰ�װ��B��Cװ��������ҩƷ��Ҫʹ��Ӧ��ʼ��Ӧ��װ��B���еIJ����� ��
Ӧ��װ��C���еIJ����� ��
��4��B�в�����˫��ϴ��������װ�ã��������� ����Ӧ��˫��ϴ�����п��ܳ��ֵ������� ��
��5��Bװ�ô����������Ե�ȱ�㣬ʹʵ���Ч�����û����������С�������ȱ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�0.1 mol/L��H2C2O4��Һ��H2C2O4��HC2O4-��C2O42-��������ռ���ʵ����������ֲ�ϵ������pH�仯�Ĺ�ϵ����ͼ��ʾ�����б�������ȷ����
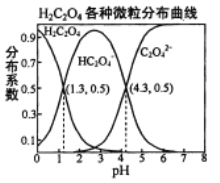
A. HC2O4-![]() H+��C2O42-��K��1��10-4.3
H+��C2O42-��K��1��10-4.3
B. �������ʵ�����NaHC2O4��Na2C2O4����ˮ����������ҺpHǡ��Ϊ4.3
C. ������HF��K��1��10-3.4��������H2C2O4��Һ���뵽����NaF��Һ���������ķ�ӦΪ��H2C2O4��F-��HF��HC2O4-
D. ��0.1 mol/L NaHC2O4��Һ����������Ũ�ȴ�С��ϵΪ��c(Na+)��c(HC2O4-)��c(H+)��c(C2O42-)��c(OH-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ǧ�ĵ��ʡ�����������ִ���ҵ��������Ҫ��;��
��.��1��Ǧ���γɶ�������������������PbO������������PbO2������Fe3O4��Pb3O4��Pb3O4��HNO3������������ԭ��Ӧ����һ���κ�һ��Ǧ������䷴Ӧ�Ļ�ѧ����ʽΪ_____________��
��.�Ժ�Ǧ����(��Ҫ��Pb��PbO��PbO2��PbSO4)Ϊԭ���Ʊ��ߴ�PbO������Ҫ�������£�

��2����������ʱ����Fe2+���£�Pb��PbO2��Ӧ����PbSO4����1molPbSO4ת�Ƶ��ӵ����ʵ���Ϊ____________mol��
��3����֪����PbO�ܽ���NaOH��Һ�У�����ƽ�⣺PbO(s)+NaOH(aq)![]() NaHPbO2(aq)�����ܽ��������ͼ��ʾ��
NaHPbO2(aq)�����ܽ��������ͼ��ʾ��

�ڴ�ƷPbO���������ʲ�����NaOH��Һ��
���������Ϣ������ɴ�ƷPbO�õ��ߴ�PbO�IJ���������ƷPbO�ܽ���һ����__________(����35%������10%��)��NaOH��Һ�У�������110�棬����ܽ��__________������Һ��ȴ�ᾧ�����ˡ�ϴ�Ӳ�����õ��ߴ�PbO���塣
��4����PbO��Ʒ�ܽ���HCl��NaCl�Ļ����Һ�У��õ���Na2PbCl2�ĵ��Һ�����Na2PbCl4��Һ����Pb��װ����ͼ��ʾ��

�������ĵ缫��ӦʽΪ_______________________��
�ڵ��һ��ʱ���Na2PbCl4Ũ�ȼ����С��Ϊ�˻ָ���Ũ����ʵ�����ʵ�ѭ�����ã���������ȡ�ķ�����_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D������ѧ��ѧ�г��������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת����ϵ���£����ַ�Ӧ�е�ˮ����ȥ������������ش��������⣺

��1����A��B��C����ɫ��Ӧ��Ϊ��ɫ��CΪ���ͷ۵ijɷ�֮һ��D�Ĺ����ŷŻ��������ЧӦ��
��B������Ϊ_______________��
����Ӧ�������ӷ���ʽ��_________________________________________��
��2����A��D��Ϊ���ʣ���AΪ���壬DԪ�ص�һ�ֺ���ɫ�����ﳣ�������ϡ�
��д��A��һ����;_________________________��
�ڷ�Ӧ�������ӷ���ʽ��_________________________________________��
�ۼ���B����Һ�������ӵķ�����________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ʵ���Ҫ���������ʵĽṹ����ش��������⡣
(1)��֪X��YΪ��������Ԫ��,��ԭ�ӵĵ�һ�����ĵ��������±���ʾ��
������/(kJ/mol) | I1 | I2 | I3 | I4 |
X | 578 | 1817 | 2745 | 11578 |
Y | 738 | 1451 | 7733 | 10540 |
Xͨ����_____�ۣ�X�ĵ縺��____Y�ĵ縺��(�>���� =����<��)��
(2)�����Ĺ��������е�����ԼΪ399kJ/mol�������±��йص����ʷ�������Ҫ��ѧ������Ϣ��˵�����峤ʱ������������Ƥ�������˺���ԭ��_______________��
���ۼ� | C-C�� | C-N�� | C-S�� |
����/(kJ/mol) | 347 | 305 | 259 |
��ɵ����ʵ���İ������е�̼ԭ�ӵ��ӻ�������__________��
(3)ʵ��֤��:KCl��MgO��CaO��TiN�����־���Ľṹ��NaCl����ṹ����(��ͼ),����3�����Ӿ���ľ������������±�:
���Ӿ��� | NaCl | KCl | CaO |
������/(kJ.mol-1) | 786 | 715 | 3401 |
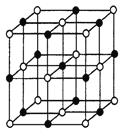
�����������Ӿ�����۵�Ӹߵ��͵�˳����_______������MgO������һ��Mg2+��Χ�������ڽ��ҵȾ����Mg2+��______����
(4)���������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�ż�¼����Խ�á������ͻ�����V2O5��CrO2�У��ʺ���¼�����ŷ�ԭ�ϵ���_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ�о���ѧϰС�������ƺ���NH![]() ��Cl����K����SO42����ֲ������Һ450 mL����Ҫ�������Һ��c(Cl��)��c(K��)��c(SO42��)��0.4 mol��L��1��ʵ�����ṩ��ҩƷ�У�NH4Cl��KCl��(NH4)2SO4��K2SO4������ˮ���ṩ��ʵ�������У���ҩ�ס���������ƽ�����ձ����ܲ��������ݽ�ͷ�ιܡ�����Ͳ����ش��������⣺
��Cl����K����SO42����ֲ������Һ450 mL����Ҫ�������Һ��c(Cl��)��c(K��)��c(SO42��)��0.4 mol��L��1��ʵ�����ṩ��ҩƷ�У�NH4Cl��KCl��(NH4)2SO4��K2SO4������ˮ���ṩ��ʵ�������У���ҩ�ס���������ƽ�����ձ����ܲ��������ݽ�ͷ�ιܡ�����Ͳ����ش��������⣺
(1)��ֲ������Һ�У�NH![]() �����ʵ���Ũ��Ϊ_____________��
�����ʵ���Ũ��Ϊ_____________��
(2)���о�С�����Ƹ�ֲ������Һʱ�������õ���ʵ��������________(����ĸ)��
A��450 mL����ƿ B��500 mL����ƿ C������250 mL����ƿ
(3)��ͬѧ��KCl��(NH4)2SO4�������ʽ������ƣ������ȡm(KCl)��____g��m[(NH4)2SO4]��___g��
(4)�����Ƹ�����Һ��������������ȷ�������д��������ʹ��������Һ��Ũ��ƫ�͵���_________(��ѡ��)��
A������Һת��������ƿ��δϴ���ձ��Ͳ�����
B�����ձ��ڵ���Һ������ƿ��ת��ʱ�����������ʹ������Һ��������ƿ
C���ý�ͷ�ι�������ƿ�м�ˮʱ����Һ�İ�Һ���������ƿ�̶���
D���ý�ͷ�ι�������ƿ�м�ˮʱ����������ƿ�̶���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com