
����Ŀ���⻯��������״���������μ�����̵����������ȡ�ʵ������NaOH�����ʵ��ˮ����(N2H4��H2O)Ϊԭ�Ͽ��Ʊ��⻯�ơ�
������ʾ��ˮ�����л�ԭ�ԣ�������ˮ���ܽ��������NaIO3��һ����������
�ش��������⣺
��1��ˮ���µ��Ʊ����йط�Ӧԭ��Ϊ��NaClO + 2NH3 = N2H4��H2O + NaCl��
������ͼװ����ȡˮ���£�������˳��Ϊ_________(������������Сд��ĸ��ʾ)��




��װ��A��������_______��
�ۿ�ʼʵ��ʱ�������������еμ�Ũ��ˮ��һ��ʱ�������B��������ƿ�еμ�NaClO��Һ���μ�NaClO��Һʱ���ܹ��������___________��
��2���⻯�Ƶ��Ʊ�
��.��������ƿ�м���8.4gNaOH��30mLˮ�����衢��ȴ������25.4g�ⵥ�ʣ���������������������60~70������Ӧ��֣�
�������������Թ�����N2H4��H2O(ˮ����)����ԭNaIO��NaIO3����NaI��Һ��Ʒ��ͬʱ�ͷ�һ�ֿ����е����壻
������������ӦҺ�м���1.0g����̿����а�Сʱ��Ȼ����Һ�����̿���룻
���������袣���������Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ò�Ʒ24.0g��
�ܲ��袡���˲��õļ��ȷ�����ˮԡ���ȣ� �ò��跴Ӧ��ȫ��������_________�����袢��IO3�����뷴Ӧ�����ӷ���ʽΪ________________________________��
�ݲ��袣 ������Һ�����̿���롱�ķ����dz��ȹ��ˡ�
�ޱ���ʵ�����Ϊ__________��ʵ�鷢�֣�ˮ����ʵ������������ֵƫ�ߣ����ܵ�ԭ����___________��
��ijͬѧ�����ƷNaI���Ƿ����NaIO3���ʡ�ȡ����������Ʒ���Թ��У���ˮ�ܽ⣬�μ���������Һ���ٵμ�����ϡ���ᣬƬ�̺���Һ�������ó�NaI�к���NaIO3���ʡ������۸�ʵ����۵ĺ�����____________��������Ϊ����д�����ӷ���ʽ������Ϊ������˵�����ɣ�
���𰸡�f a b c d e (ab˳��ɻ���) ��ֹ������ȫƿ ����μ�NaClO��Һ��������NaClO��Һ����ˮ���£����Ͳ��� �����������Һ����ɫ 2IO3��+ 3N2H4��H2O =3N2��+2I-+9H2O 80% ˮ��������ˮ�е��ܽ�����Ӧ ������I�������Ի����б�O2������I2��ʹ���۱���
��������
(1)���ɷ�Ӧԭ��NaClO+2NH3=N2H4![]() H2O+NaCl��֪,����Dװ�ò�������,Ϊ��ֹ��������,�������װ��A��,Ȼ����ͨ��c���ܽ���װ��B��,Ȼ����������백��Ӧ,����İ����Cװ������;������˳��Ϊfabcde;��ȷ��:fabcde����װ��A�������Ƿ�ֹ������ȫƿ;��ȷ��:��ֹ������ȫƿ��
H2O+NaCl��֪,����Dװ�ò�������,Ϊ��ֹ��������,�������װ��A��,Ȼ����ͨ��c���ܽ���װ��B��,Ȼ����������백��Ӧ,����İ����Cװ������;������˳��Ϊfabcde;��ȷ��:fabcde����װ��A�������Ƿ�ֹ������ȫƿ;��ȷ��:��ֹ������ȫƿ��
�۹���μ�NaClO��Һ,����NaClO������ȫ��Ӧ,������NaClO��Һ�ܹ�������Ӧ������ˮ����,���½��Ͳ���;��ȷ��: ����μ�NaClO��Һ��������NaClO��Һ����ˮ���£����Ͳ�����
(2)�ܱ���60~70������·�����Ӧ,��˿��Բ���ˮԡ���ȵķ���;�����������������Һ��ַ�Ӧ������ɫ��NaIO��NaIO3,������ȫ����ʧ;���Ըò��跴Ӧ��ȫ�������������������Һ����ɫ;N2H4![]() H2O���л�ԭ��,�ܹ���I03-��ԭΪ������,��-2�۵ĵ�Ԫ�ر�����Ϊ����,���ӷ���ʽΪ: 2IO3��+ 3N2H4��H2O =3N2��+2I-+9H2O;��ȷ��:�����������Һ����ɫ; 2IO3��+ 3N2H4��H2O =3N2��+2I-+9H2O��
H2O���л�ԭ��,�ܹ���I03-��ԭΪ������,��-2�۵ĵ�Ԫ�ر�����Ϊ����,���ӷ���ʽΪ: 2IO3��+ 3N2H4��H2O =3N2��+2I-+9H2O;��ȷ��:�����������Һ����ɫ; 2IO3��+ 3N2H4��H2O =3N2��+2I-+9H2O��
��8.4gNaOH��25.4g���ʵⷴӦ���������ƹ���,�ⵥ�ʷ�Ӧ��ȫ,����������Ʒ�����ӦΪ3I2+6NaOH=5NaI+NaIO3+3H2O,�����ɵ�NaI������Ϊ25.4![]() 750/762=25g,���ɵ�NaIO��N2H4
750/762=25g,���ɵ�NaIO��N2H4![]() H2O��Ӧ���õ�NaI,��ӦΪ3N2H4
H2O��Ӧ���õ�NaI,��ӦΪ3N2H4![]() H20+2NaIO3=2NaI+3N2
H20+2NaIO3=2NaI+3N2![]() +9H2O,��6I2
+9H2O,��6I2![]() 2NaIO3
2NaIO3![]() 2NaI�ò����ɵ�NaI����Ϊ25.4
2NaI�ò����ɵ�NaI����Ϊ25.4![]() 300/1524=5g,�����������ɵ�NaIΪ25g+5g=30g,����,����ʵ��IJ���Ϊ24/30
300/1524=5g,�����������ɵ�NaIΪ25g+5g=30g,����,����ʵ��IJ���Ϊ24/30![]() 100%=80%;ˮ����ʵ������������ֵƫ��,ˮ���»�ԭ�Խ�ǿ,������ˮ�е��ܽ�����Ӧ;��ȷ��:80%;ˮ��������ˮ�е��ܽ�����Ӧ��
100%=80%;ˮ����ʵ������������ֵƫ��,ˮ���»�ԭ�Խ�ǿ,������ˮ�е��ܽ�����Ӧ;��ȷ��:80%;ˮ��������ˮ�е��ܽ�����Ӧ��
�߸÷���������,�����Ӿ���ǿ��ԭ��,�����������±����������ɵⵥ��,����ʹ������Һ����;��ȷ��:������1-�����Ի����б�O2������I2��ʹ���۱�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������̫���ܵ���е�����̫���ܵ�ء��ྦྷ��̫���ܵ�ء�GaAs̫���ܵ�ؼ�ͭ�������ȶ���Ԫ�ع��ɱ�Ĥ̫���ܵ�ء��ش��������⣺
(1)��̬��ͭ����(Cu+)�ļ۲�����Ų�ʽΪ__________��
(2)�顢���ǵ������ڵ�����Ԫ�أ���֪��ĵ�һ������(947kJ��mo1��1)������(941k]��mol��l)�����ԭ�ӽṹ�ĽǶȼ��Խ���__________��
(3)H2O�ķе����H2Se�ķе㣬��ԭ����__________��
(4)GaCl3��AsF3�ķ������幹�ͷֱ���__________��__________��
(5)����(H3BO3)�������ܵ����H+����ˮ�����һ��OH������[B(OH)4]���������������ԡ�
��[B(OH)4] ����Bԭ�ӵ��ӻ�����Ϊ__________��
��[B(OH)4] ���ĽṹʽΪ__________��
(6)���ʯ�ľ�����ͼ�����Թ�ԭ�Ӵ�����ʯ�����е�̼ԭ�ӣ���õ�����裻�������ʯ������һ���̼ԭ�ӻ��ɹ�ԭ�ӣ���̼����ԭ�ӽ��棬���õ�̼���辧��(���ɰ)��

�ٽ��ʯ������衢̼������۵��ɸߵ��͵�����˳����__________(�û�ѧʽ��ʾ)
�ڽ��ʯ�ľ�������Ϊapm(lpm=10��12m)��1cm3�����ƽ������Ϊ__________g(ֻҪ������ʽ�������ӵ�������ֵΪNA)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڹ��˵������ȷ���ǣ� ��
A.���ڵؿ��еĺ�����������
B.������Ȼ���м��л���̬����������̬
C.�������ཫ̫����ת��Ϊ���ܵij��ò���
D.�赥�ʵĵ����������ڵ���;�Ե��֮��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ�������������:
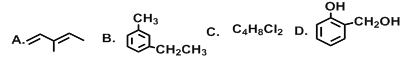

��1��G��ͬһֱ���ϵ�̼ԭ����Ϊ___________��
��2�������л��ﻥΪͬ���칹�����__________(����ĸ)��
��3��A��ϵͳ����Ϊ______________������Cl2��1:1������Ӧʱ������_________�֣�
��4�������л�����������Ʒ�Ӧ����_________������ĸ��������̼�����Ʒ�Ӧ����______������ĸ����B�����Ը�����ط�Ӧ�IJ�����______________(��ṹ��ʽ)��
��5��C��ͬ���칹����________�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��C2H4���ǻ����л�����ԭ�ϡ���A�Ʊ���Ҫ�м���G·�ߣ����ַ�Ӧ������ȥ��������ʾ��

��֪����1��
�ش��������⣺
��1��B�ĵ���ʽ_________��C�к��еĹ����ŵ�������______________��
��2����Ӧ��1���ͣ�4���ķ�Ӧ���ͷֱ���_____________��_____________��
��3����Ӧ��2���ķ�Ӧ������____________��
��4����֪G�ķ���ʽΪC8H12O3����ṹ��ʽΪ_______________��
��5��д����Ӧ(3)�Ļ�ѧ��Ӧ����ʽ_________________________________��
��6��X��C��Ϊͬϵ���ұ�C��һ��̼����X��___�ֽṹ����֪˫�����ǻ����ȶ������ǣ�
��7�����������ϳ�·�ߣ����һ������Ȳ�ͱ�ͪΪ��ʼԭ���Ʊ�2-��-1,3����ϩ�ĺϳ�·��__________����д��ʽ��ͼ�� ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ�IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ����Ҫ��־��
I����8.96L (��״��)��ϩ������Ļ������ͨ��������������Ȼ�̼��Һ�У���ַ�Ӧ��������Ȼ�̼��Һ����������8.4g����ԭ������������ϩ����������ʵ���֮��Ϊ______________��
II����֪��ϩ�ܷ�������ת����
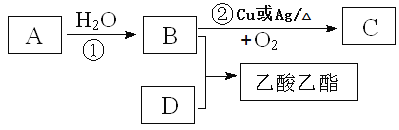
��д��B��D�������й����ŵ����ƣ�B____________________��D___________________��
��д����ط�Ӧ�Ļ�ѧ����ʽ��
��_________________________________ ��Ӧ���ͣ�________________
��__________________________________����Ӧ���ͣ�_________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������йؽ������仯�����˵����ȷ����
A.��������������Һ��Ӧ����Al(OH)3��H2O
B.���ڿ�����ȼ�����ɵ���ɫ��Na2O
C.���ڸ�������ˮ������Ӧ����Fe2O3��H2
D.̼������Һ������������Һ��Ӧ����NaOH��CaCO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(NaNO2)��һ�ֹ�ҵ�Σ���������������Ӧ�ù㷺����ۿ���ʳ��������ζ����һ�ֳ��õķ�ɫ���ͷ���������ʹ�ù�����ʹ���ж���ijѧϰС��ͨ������װ��(�гּ�����װ����ȥ)��ȡNaNO2��Ʒ������Ʒ�������вⶨ
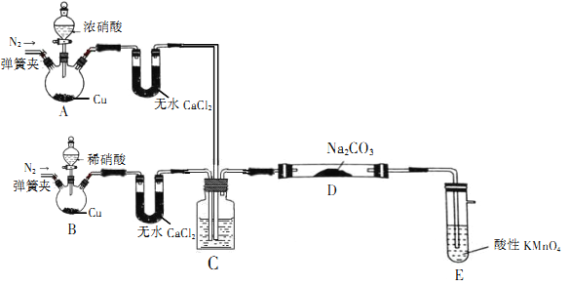
��С���Ȳ�������֪��NO��NO2�ܱ����Ը����������ΪNO3����
(1)װ��A������ʢ��Ũ�������������Ϊ__________��װ��D�з����Ļ�ѧ��Ӧ����ʽΪ___________��
(2)��װ��C����װ��Һ���������������____________________��
��������Ϊ____________________��
(3)װ��E��������____________________��
(4)Ϊ�ⶨ�Ƶ���Ʒ��NaNO2�ĺ�������ʵ����������KMnO4����Һ���ữ��Ӧѡ��__________(�ѧʽ)���ữKMnO4��Һ��
(5)��֪����������Һ�У�NO2���ɽ�MnO4����ԭΪMn2+��Ϊ�ⶨ��Ʒ���������Ƶĺ�������С���ȡ4.0g��Ʒ����ˮ���Ƴ�250mL��Һ��ȡ25.00mL��Һ����ƿ�У���0.100mol��L��1������KMnO4��Һ���еζ�������20.00mL����KMnO4��Һ(���ʲ���Ӧ)���ζ�����������KMnO4��Һ��������____________________��������Ʒ��NaNO2����������Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Լ��ɼ��𱽡�1-��ϩ�����ӡ��Ҵ����� (������)
A.ˮB.Ũ��ˮC.���Ȼ�̼D.FeCl3��Һ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com