
����Ŀ����.Ϊ̽������ͭ����(CuSO4��xH2O)���ȷֽ�����ò�����ʵ��װ����ͼ��ʾ������ʵ������Ϊ��A����ɫ������ɰ�ɫ��ĩ�������������ձ�ɺ�ɫ��B�в�����ɫ������D����Һ��ɺ�ɫ��(ϴ��ƿ���Լ�������)

(1)�����Ʋ������ͭ�������շֽ���������_______________________________��
(2)D�еķ�Ӧ���������У�д����һ����Ӧ�����ӷ���ʽ_____________________��
II.�ⶨ����ͭ����(CuSO4��xH2O)�нᾧˮx��ֵ��ʵ��װ�ú������£�ȡ����ͭ����7.23 g����Ӳ���Թ��У���ͨN2�ų���ϵ�ڿ������ƾ���Ƹ��¼��ȳ�֣���A����ɫ�������ձ�ɺ�ɫ��ֹͣ���ȣ��ٴι���N2��װ����ȴ�����¡�(ϴ��ƿ���Լ�������)
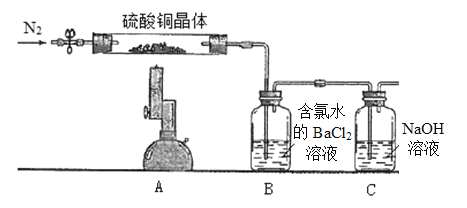
(1)ȡB�а�ɫ����������ϴ�Ӹ�������ù���6.99 g��������ɵ�CuSO4��xH2O��x=__________���ٴι���N2��Ŀ����____________________��
(2)ijͬѧ�����Ҫ�ⶨ�����нᾧˮx��ֵ��Ҳ�ɽ�Bװ����װ��Ũ�����ϴ��ƿ�滻�����ղ�Ũ�������ؼ��ɼ���õ���������۸�ͬѧ�ķ����Ƿ���У�(��������У���˵������)_____________________________
���𰸡�CuO��SO3��SO2��O2��H2O 4Fe2++O2+4H+ = 4Fe3++2H2O 4.5 ʹ�ֽ����������ȫ����ϴ��ƿ���Լ����� �����У�SO3Ҳ���ܽ���Ũ�����У�Ũ��������ز�ֹֻ��ˮ������
��������
I����ʵ���Ŀ����̽������ͭ����(CuSO4��xH2O)���ȷֽ�����ò��A����ɫ������ɰ�ɫ��ĩ�������������ձ�ɺ�ɫ��˵���������ȷֽ������CuO��B������BaCl2��Һ������ɫ��������ֽ��������SO3��D����Һ��죬˵��Fe2+������ΪFe3+���������������������ʣ����������غ㶨�ɿ�֪��������������ΪO2����ֽ��������SO2���ɣ�CuSO4�У�OΪ-2�ۣ����õ�O2����Ҫ�����ϼۣ�����S�Ļ��ϼ�һ�����ͣ�����SO2��������B�е�������Ʒ����Һ��ɫ��E�����������ն�����к����壬��ֹ��Ⱦ������
II������ʵ��I�Ƴ�����ͭ���������SO2��SO3��B�к���Cl2��BaCl2��Cl2��ˮ�п��Խ�SO2����ΪSO42-����CuSO4�е�Sȫ��ת��Ϊ��BaSO4�������ͨ��BaSO4������������x��ֵ��
I����1����������CuSO4���ȷֽ�����IJ�����CuO��SO2��SO3��O2�����ھ�����ԣ����ȷֽ���ﻹ��H2O���ʿ��Ʋ������ͭ�������շֽ���������CuO��SO3��SO2��O2��H2O��
��2��D�У�Fe2+�ȱ�O2����ΪFe3+��Fe3+�ٺ�SCN-�������Ѫ��ɫ���ʣ��漰�����ӷ�ӦΪ��4Fe2++O2+4H+=4Fe3++2H2O��Fe3++3SCN-=Fe(SCN)3��
II����1�����������֪��n(BaSO4)=![]() =0.03mol����n(CuSO4��xH2O)=0.03mol��������0.03mol��(150+18x)g/mol=7.23g�����x=4.5���ٴ�ͨ��N2��Ŀ����ʹ�ֽ����������ȫ����ϴ��ƿ���Լ����գ�
=0.03mol����n(CuSO4��xH2O)=0.03mol��������0.03mol��(150+18x)g/mol=7.23g�����x=4.5���ٴ�ͨ��N2��Ŀ����ʹ�ֽ����������ȫ����ϴ��ƿ���Լ����գ�
��2�������У��ֽ����SO3�����ܽ���Ũ�����У�Ũ��������ز�ֹֻ��ˮ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ҫ��ش��������⡣
��CaBr2����H2O����NH4Cl����H2O2����Na2O2����Ca(OH)2����HClO����CO2
(1)д��Na2O2�ĵ���ʽ________�����ڵĻ�ѧ��������________________��
(2)д��HClO����ʽ________________,д��CO2�Ľṹʽ_______��
(3)���й��ۼ������ӻ�������_______(�����)
(4)�õ���ʽ��ʾCaBr2���γɹ���_________________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CH3OH����Ҫ�Ļ���ԭ��,��ҵ����CO��H2�ڴ��������ºϳ�CH3OH,�䷴ӦΪ:CO��g��+2H2��g��CH3OH��g������n(CO):n(H2)=1:2�����ܱ������г��뷴Ӧ��,���ƽ��ʱ�������CH3OH����������ڲ�ͬѹǿ�����¶ȵı仯��ͼ��ʾ������˵����,��ȷ����
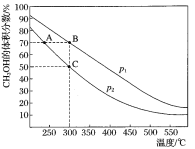
A. p1<p2
B. �÷�Ӧ�Ħ�H>0
C. ƽ�ⳣ��:K(A)=K(B)
D. ��C��ʱ,COת����Ϊ75%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����㻯����F���л���������Ҫԭ�ϣ�Ҳ���ƶ����ܹ��������ơ�
��֪����������������ʱ���������ȡ�����������������ڶ�λ�ϣ����������Ȼ�ʱ���������ȡ���������Ȼ��ļ�λ�ϡ�
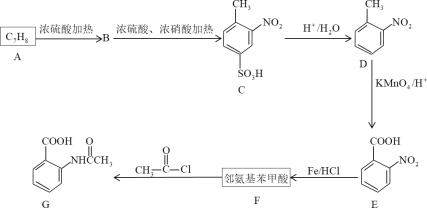
��1��D���ʵ�����Ϊ___��G�й����ŵ�����Ϊ___��___��
��2��A��F�Ľṹ��ʽ�ֱ�Ϊ___��___��E����F�ķ�Ӧ������___��
��3��д��A��B�����϶�ĸ�����Ľṹ��ʽ___��������δ���üױ�ֱ�������ķ�����ȡD�����Ǿ���������Ӧ���Ƶ�D��Ŀ����___��
��4��F��һ���������ܷ����ۺϷ�Ӧ����д���仯ѧ��Ӧ����ʽ___��
��5��F��ͬ���칹���У�����ֱ�����ڱ����ϡ����ܷ���������Ӧ�ķ����廯���ﹲ��___�֣���������ṹ����
��6�����Լױ�Ϊԭ�ϣ�д���ϳɼ䰱�������������ͼ___�����Լ���ѡ�����ϳ�����ͼ��ʾ����ʾ����CH2=CH2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2OH
CH3CH2OH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���״�����Ϊ2l���͵�����ȼ�ϣ���ҵ��ͨ�����з�Ӧ��͢���CH4��H2OΪԭ�����Ʊ��״���
��1����1.0molCH4��2.0molH2O��g��ͨ���ݻ�Ϊ10L�ķ�Ӧ�ң���һ�������·�����Ӧ��CH4��g��+H2O��g��![]() CO��g��+3H2��g����CH4��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��
CO��g��+3H2��g����CH4��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��
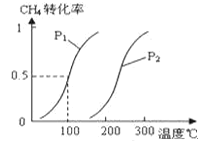
����֪100��ʱ�ﵽƽ�������ʱ��Ϊ5min������H2��ʾ��ƽ����Ӧ����Ϊ______��
���������������������������¶ȣ���ѧƽ�ⳣ����_______����������������С����������������
��ͼ�е�P1_____P2����������������������=������100��ʱƽ�ⳣ��Ϊ________��
�ܱ��ַ�Ӧ��ϵΪ100�棬5min�����������г���H2O��H2��0.5mol����ѧƽ�⽫��_____�ƶ���������������������������������
��2����ѹǿΪ0.1MPa�����£���amolCO��3amol H2�Ļ�������ڴ������������Է�������Ӧ��
CO��g��+2H2��g��![]() CH3OH��g��
CH3OH��g��
�ٸ÷�Ӧ�ġ�H_____0����S_____0����������������������=������
���������ݻ����䣬���д�ʩ�������COת���ʵ���______��
A�������¶�
B����CH3OH��g������ϵ�з������
C������He��ʹ��ϵ��ѹǿ����
D���ٳ���1molCO��3molH2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ʵ���֮��Ϊ1:3�ĵ�����������������ܱ������У��ⶨ��ͬ�¶ȡ�ѹǿ��ƽ�������а������ʵ��������������ͼ��ʾ������˵������ȷ���ǣ� ��
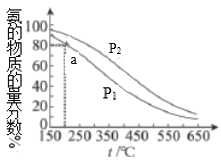
A.![]()
B.�÷�Ӧ![]()
C.![]() �㣬
�㣬![]() ��ת����Ϊ
��ת����Ϊ![]()
D.�ϳɰ���ҵʵ�����˹��̵�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NA��ʾ�����ӵ���������ֵ�����е�˵���У���ȷ���ǣ� ��
A. 4.6g��������ԭ����ȫ��ΪNa+ ����ʱ��ʧȥ�ĵ�����Ϊ0.1NA
B. NA ������������NA ���������ӵ�������Ϊ8�U1
C. 0.2 NA�����������19.6g���ᣨ��Է���������98��������ͬ����ԭ����
D. 22.4L�ĵ��������е�ԭ����Ϊ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���¶��£����淴ӦX��g����3Y��g��![]() 2Z��g���ﵽ��ѧƽ��״̬�ı�־��
2Z��g���ﵽ��ѧƽ��״̬�ı�־��
A.Z���������ʺ�X�ķֽ��������
B.��λʱ��������nmolX��ͬʱ������3nmolY
C.X��Y��Z��Ũ�Ȳ��ٱ仯
D.X��Y��Z�ķ��Ӹ���֮��Ϊ1��3��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��ӦA2��g����B2��g��===2AB��g���������仯��ͼ��ʾ�������й�������ȷ����
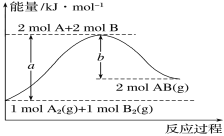
A.ÿ����2molAB��g������bkJ����
B.����1molA��A����1molB��B�����ų�akJ����
C.�÷�Ӧ�з�Ӧ��������������������������
D.��Ӧ����H������a��b��kJ��mol��1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com