



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
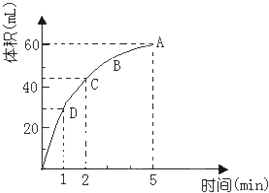 Ϊ���о�MnO2��˫��ˮ��H2O2���ķ�Ӧ���ʣ�ijѧ������������MnO2��ĩ��50mL�ܶ�Ϊ1.1g/cm3��˫��ˮ��Һ�У�ͨ��ʵ��ⶨ���ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ������ͼ�ش��������⣺
Ϊ���о�MnO2��˫��ˮ��H2O2���ķ�Ӧ���ʣ�ijѧ������������MnO2��ĩ��50mL�ܶ�Ϊ1.1g/cm3��˫��ˮ��Һ�У�ͨ��ʵ��ⶨ���ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ������ͼ�ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
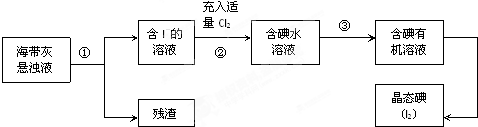
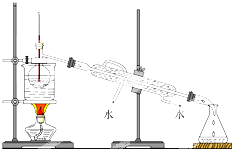
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������Ԫ����ԭ�Ӱ뾶��С����F |
B�����ݦм��ijɼ������ж�C-C�ļ�����  ���ܵĹ�ϵ��˫���ļ���С�ڵ����ļ��ܵ�2�� ���ܵĹ�ϵ��˫���ļ���С�ڵ����ļ��ܵ�2�� |
| C��Ԫ�ص縺��ԽС��Ԫ�طǽ�����Խǿ |
| D����n���ڵ�n�����Ԫ�ؾ�Ϊ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� I2��g��+H2��g��?2HI��g�� |
B�� CH3COOH?H++CH3COO-��������Һ����仯�� |
C��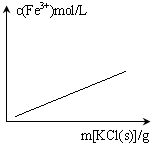 FeCl3+3KSCN?Fe��SCN��3+3KCl������Һ������仯�� |
D��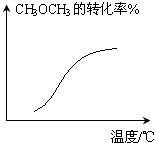 CH3OCH3��g��+3H2O��g��?6H2��g��+2CO��g��-Q����ѹ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ѡ�� | W | X | Y | Z |
| A | CH3COOC2H5 | CH3COOH | Na2CO3��Һ | ��Һ |
| B | CO2 | SO2 | ����Na2CO3��Һ | ϴ�� |
| C | NaCl���� | I2 | CCl4 | ��Һ |
| D | Na2CO3��Һ | NaHCO3��Һ | Ca��OH��2��Һ | ���� |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ �� ���� |
| B��1g H2��2g H2 |
| C��3molC2H2��1molC6H6 |
| D�������Ǻ��� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com