
����Ŀ�����仯����������������Ӧ�ù㷺���ش��������⣺
(1)��立���һ�ֻ�ѧ����,����ʽ�� NH4Fe(SO4)2��12H2O,������ˮ��,��Һ�е�����Ũ�ȴ�С��ϵΪ__________________��
(2)��֪ij��Һ�к��� CO32����SO42��������,ȡһ�����ĸ���Һ,�����еμ�BaCl2��Һ���� CO32����ʼ�� ��ʱ����Һ��c(CO32-)/c(SO42-)Ϊ_______________��(��֪ Ksp(BaSO4 )��1.0��10��10 ��Ksp(BaCO3)��2.5��10��9 )
(3)��֪��S2Cl2(l)��Cl2(g)��2SCl2(l) ��H����50.2kJ��mol��1 ������ 1molCl��Cl����1molS��S���ֱ���Ҫ���� 243kJ��268kJ ������������� 1mol S��Cl����Ҫ���յ�����Ϊ____kJ��
(4)�� NaOH ��Һ���������е� SO2,�����õ� Na2SO3 ��Һ���е��,�����Ʊ�H2SO4����ԭ������ͼ��ʾ(�缫����Ϊʯī)��
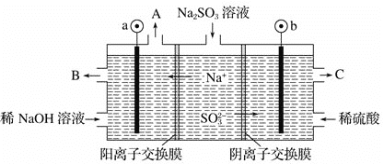
�����ĵ缫��ӦʽΪ______________________�����п�ѭ��ʹ�õ�������________��
���𰸡�c(SO42��)>c(NH4��)>c(Fe3��)>c(H��)>c(OH��) 25 280.6 SO32����2e����H2O=SO42����2H�� NaOH
��������
��1���������NH4Fe(SO4)2��12H2O����ˮ�γɵ���Һ�У�Fe3����NH4��ˮ����Һ�����ԣ�Fe3����ˮ��̶ȴ���NH4����ˮ��̶�Խ������Ũ��ԽС���ݴ˽��
��2������c(CO32-)/c(SO42-)= Ksp(BaCO3)/ Ksp(BaSO4 )������
��3����Ӧ���ʱ���ڷ�Ӧ��ļ��ܼ�ȥ������ļ��ܣ������1mol S��Cl����Ҫ���յ�����Ϊx���з��������
��4��ͼ����������b���ƶ�����bΪ����������b����SO32��������ʧ��������SO42�����ݴ˽����
��1���������NH4Fe(SO4)2��12H2O����ˮ�γɵ���Һ�У�Fe3����NH4��ˮ����Һ�����ԣ�Fe3����ˮ��̶ȴ���NH4����ˮ��̶�Խ������Ũ��ԽС������c(NH4��)>c(Fe3��)����������Ӳ�ˮ�⣬Ũ�����������Һ������Ũ�ȹ�ϵc(SO42��)>c(NH4��)>c(Fe3��)>c(H��)>c(OH��)��
��ˣ�������ȷ������c(SO42��)>c(NH4��)>c(Fe3��)>c(H��)>c(OH��)��
��2������c(CO32-)/c(SO42-)= Ksp(BaCO3)/ Ksp(BaSO4 )��֪��c(CO32-)/c(SO42-)=![]() 25��
25��
��ˣ�������ȷ������25��
��3����Ӧ���ʱ���ڷ�Ӧ��ļ��ܼ�ȥ������ļ��ܣ������1mol S��Cl����Ҫ���յ�����Ϊx����2x+268 kJ+243 kJ-4x=-50.2 kJ�����x=280.6 kJ��
��ˣ�������ȷ������280.6��
��4��ͼ����������b���ƶ�����bΪ����������b����SO32��������ʧ��������SO42������缫����ʽΪ��SO32����2e����H2O=SO42����2H����aΪ�������������ŵ�����Ϊ����������������B��������������Ũ�Ƚϴ������������Һ�����������ն������ʿ�ѭ��ʹ�õ�������NaOH��
��ˣ�������ȷ������SO32����2e����H2O=SO42����2H�� ��NaOH��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڷ��ӡ�ԭ�ӡ����ӵ�˵����������ǣ� ��
�ٶ�����̼��̼ԭ�Ӻ���ԭ�ӹ��ɣ��ڵ�������������Һ�������������Һ����˵�����Ӽ�϶�����˸ı䣻�۷����DZ����������ʵ�һ�������ܷ���һ����ԭ�Ӵ�ԭ������С�����ӣ������ٷ�
A.�٢ۢܢ�B.�ڢۢܢ�
C.�٢ڢۢܢ�D.�٢ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о���ѧϰС�������һ��ʵ����֤Ԫ�������ɣ�
��ͬѧ���������ͼװ����һ�������Ԫ�ص���̼����ǽ�����ǿ���ıȽϡ�
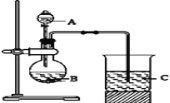
��1����ͬѧ�����Ӻ���������ҩƷ֮ǰҪ���װ�������ԡ����ȹر�_____�������������ձ�Һ�����£���_____�����C��______����˵��________
��2��Ҫ֤������̼����ǽ�����ǿ������A�м�________��Һ��B�м�____��Һ��C�м�________��Һ�����۲쵽C��__________��������ʦ��Ϊ������������֤�����߷ǽ�����ǿ��������������������_______��
��3��Ϊ�����������⣬Ӧ��B��C֮������һ��ʢ������____��ѡ��������ĸ��A��Ũ���� B��ŨNaOH��ҺC������Na2CO3��Һ D������NaHCO3��Һ����ϴ��װ�ã��Ľ���C�з�����Ӧ�����ӷ���ʽ��__________ ��
��ͬѧ���������ͼװ������֤±��Ԫ�����ʵĵݱ���ɣ�A��B��C�����ֱ���մ��NaBr��Һ������ʪ�����KI��ֽ��ʪ���ֽ��
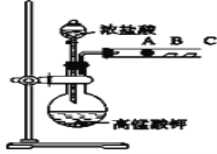
��1����д��Ũ�����������ط�Ӧ�����ӷ���ʽ_________________
��2��A������ɫ��_______����˵���ǽ�����Cl��Br����NaBr��KI�Ļ����Һ�У�ͨ��������Cl2��ַ�Ӧ��������Һ���ɲ����գ����õ���������_________
��3����ͬѧ���ô�ʵ��֤��±�ص��������ԣ�Cl2��Br2��I2������Ϊ������____���������������������������___________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڹ輰�仯����������������
A.SiO2���ȶ��������е��ᶼ����ӦB.ˮ������觵���Ҫ�ɷֶ���SiO2
C.�մɡ�������ˮ��ǹ����β�ƷD.ˮ������һ�ֳ��õĿ��コ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��F��GΪԭ��������������Ķ���������Ԫ�ء�B��C��D������A�γ�10���ӷ��ӣ�E���ʿ�����Ұ�⺸�Ӹֹ��ԭ�ϣ�F��Dͬ���塣
(1)D��E��F�����Ӱ뾶�ɴ�С��˳��Ϊ_______________________(�����ӷ���)��
(2)F��G�γɵ�һ�ֻ������������ԭ�Ӿ�Ϊ8�����ȶ��ṹ���û�������ˮ��Ӧ����F���ʡ�F����ۺ������G���⻯��÷�Ӧ�Ļ�ѧ����ʽΪ___________________��
(3)C�ֱܷ���A��D��ԭ�Ӹ�����1:2�γɻ������Һͱ����ҵĽṹʽΪ__________��������,Һ�������������Ӧ������������Ⱦ������,�÷�Ӧ�����������뻹ԭ��������ʵ���֮��Ϊ________________��
(4)��ȡ100 mL1 mol/L��E���Ȼ�����Һ,�����м���1 mol/L NaOH��Һ������3.9g������������NaOH��Һ�������Ϊ_________________________mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ƽ����ϵmA(g)��nB(g)![]() pC(g)��qD(g) ��H��0�����н����д������( )
pC(g)��qD(g) ��H��0�������������( )
A. ��ƽ��ʱ��A��B��ת������ȣ�˵����Ӧ��ʼʱ��A��B�����ʵ���֮��Ϊm��n
B. ���¶Ȳ��䣬�������������С��ԭ����һ�룬�ﵽ��ƽ��ʱA��Ũ��Ϊԭ����1.8������m��n > p��q
C. ��m��n = p��q����������a mol�����ƽ����ϵ���ټ���a mol��B���ﵽ��ƽ��ʱ������������ʵ�������2a
D. ���¶Ȳ�����С����������ﵽ��ƽ��ʱѹǿ����ԭ����2���������һ��С��ԭ����1/2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о���ѧϰС����������װ��̽�������Ͱ���֮��ķ�Ӧ���������A��F�ֱ�Ϊ��������������ȡ����װ�ã�CΪ������������������İ���������Ӧ��װ�á���ش��������⣺

(1)ʵ����ͨ��������ȡ�����Ļ�ѧ����ʽΪ ________________________��
(2)ʵ�����ռ��Ʊ��õ��İ����ij��÷�����______________________��
(3)��ΪB�����߿���ѡ����ܺ��ʵ�װ���Լ���Ӧ���Լ�����______����š�
A.���θ����װ��ʯ�� B.ϴ��ƿװŨ����
C.���θ����װ������ D.���θ����װ��������
(4)װ��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ��һ�������ǿ�������Ҫ�ɷ�֮һ��д����Ӧ�Ļ�ѧ����ʽ�� __________________����1molNH3����������ת�Ƶĵ�����ĿΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���������ʵ�����������Ũ���ᷴӦ�IJ���ش��������⣺
��.������·�����������������е�CO2��SO2��H2O��
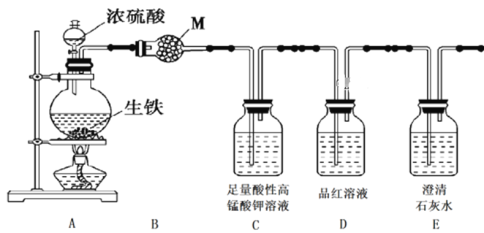
��1�������B��M�Ļ�ѧʽ��______________________��
��2��Cװ�õ�������_________________________________________________��
��3����֤���ж�����̼���ɵ�ʵ��������_______________________________��
��4����������Ũ���ᷴӦ��Ϻ�����ƿ�е���Һ����Fe3�����Լ��ǣ�________________��
��.�ÿ�����ͬѧ��Ϊ������Ũ���ᷴӦ�������������ɡ�Ϊ����֤��һ���룬ѡ������������ҩƷ���������װ�ã��������ʵ����
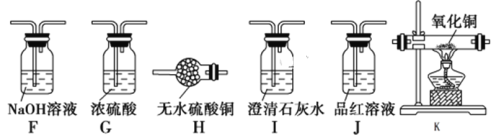
��5������ʵ����Ƶļ�Լ��ԭ�������������ң���������Ϊ______________��
��6����֤�����������ɵ�ʵ��������___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ����Ʒ���һ�����е��ǣ� ��
A.��ˮ���𱽺����Ȼ�̼
B.�ý��½ᾧ�ķ�����ȥKNO3�л���������NaCl
C.�÷�Һ�ķ������������Ȼ�̼��Һ
D.�þƾ���ȡ��ˮ�еĵ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com