
����Ŀ���±������������г��������ʣ������г������ǵ�(��Ҫ���ɷ֡�
��� | �� | �� | �� | �� | �� | �� | �� |
���� | �ƾ� | ���� | ��� | ʳ�� | ͭ���� | �������� | �մ� |
��Ҫ�ɷ� | CH3CH2OH | CH3COOH | NaOH | NaCl | Cu | SO2 | Na2CO3 |
��1������Ա��Т����ߵ���Ҫ�ɷֽ��з���(���ţ������ڵ���ʵ���______�����ڷǵ����_______��
��2�������ڵ�ˮ��Һ��߷�Ӧ�����ӷ���ʽ______________________��
��3��ijͬѧ�âݺ�Ũ���Ṳ�����Ʊ��ޣ���ѧ����ʽΪ��Cu+2H2SO4(Ũ��![]() CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O
�����õ����ű������ת�Ƶ����____________��
��ŨH2SO4���ֳ����������ǣ�_______��������ת��0.1molʱ�����������������ʵ���Ϊ_______��
��4����ͼ��ʾijͬѧ����480mL 0.5mol/L ��NaOH��Һ�IJ��ֲ���ʾ��ͼ�������д������_______���������������Ƶ���Һ��Ҫ���Ũ��Ҫ_________ (����ƫ��������ƫ����������Ӱ������������Ӧ��ȡNaOH________g��

���𰸡��ڢۢܢ� �٢� 2CH3COOH+CO32-=2CH3COO-+CO2��+H2O ![]() ���ԡ������� 0.05moL C ƫ�� 10.0
���ԡ������� 0.05moL C ƫ�� 10.0
��������
��1�����ڵ���ʵ��д��ᡢ��ʳ�Ρ��մ�ѡ�ڢۢܢߣ����ڷǵ���ʵ��оƾ���������������ѡ�٢ޣ���2��������̼���Ʒ�Ӧ�ķ���ʽΪ��2CH3COOH+CO32-=2CH3COO-+CO2��+H2O����3����������ԭ��Ӧ�е���ת������ı�ʾ���õ����ű�ʾ��������£�![]() ����ŨH2SO4���ֳ����������ǣ����ԡ������ԣ����ݷ�Ӧ����ʽ��֪��������ת��0.1molʱ�����������������ʵ���Ϊ
����ŨH2SO4���ֳ����������ǣ����ԡ������ԣ����ݷ�Ӧ����ʽ��֪��������ת��0.1molʱ�����������������ʵ���Ϊ![]() =0.05mol����4������ʱ�۾���̶�����ƽ�����ܸ��ӻ����ӣ�����ʱ���۾����ӿ̶��ߣ���Һ��Һ�泬���̶��ߣ���Һ�����ƫ��Ũ��ƫС���ʴ�Ϊ��C�� ƫ�ͣ�����480mL 0.5mol/L ��NaOH��Һʱ������û�иù�������ƿ����������500mL 0.5mol/L ��NaOH��Һ��Ӧ��ȡNaOH 0.5L��0.5mol/L��40g/mol=10.0g��
=0.05mol����4������ʱ�۾���̶�����ƽ�����ܸ��ӻ����ӣ�����ʱ���۾����ӿ̶��ߣ���Һ��Һ�泬���̶��ߣ���Һ�����ƫ��Ũ��ƫС���ʴ�Ϊ��C�� ƫ�ͣ�����480mL 0.5mol/L ��NaOH��Һʱ������û�иù�������ƿ����������500mL 0.5mol/L ��NaOH��Һ��Ӧ��ȡNaOH 0.5L��0.5mol/L��40g/mol=10.0g��


 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A. Na2O2���������ӵĵĸ�����Ϊ1��1
B. Ԫ�����ڱ��У�����Ԫ�������������ǵڢ�B��
C. NaCl��HCl����ʱ���˷���������ȫ��ͬ
D. ����������һ�������ӻ������������Ԫ�صĻ������п��������ӻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ij��Һ��ֻ���ܺ���Fe2����Mg2����Cu2����NH4+��Al3����Cl����OH����CO32����������һ�ֵ���ɫ���岢����ʱ���д̼�������ų��Ͱ�ɫ�������������뵭��ɫ��������ʵ����������꣩�������ij����Ͳ�����������ʵ����������꣩�Ĺ�ϵ����ͼ��ʾ���õ���ɫ��������ɫ��Ӧʵ���Ի�ɫ����֪��Һ�к��е�������________________���������ӵ����ʵ���Ũ��֮��Ϊ____________�����ӵĵ���ɫ������______________��

��ʵ���Ҳ��õζ����ⶨijˮ�����������κ�����

��1���ζ�ʱ��KIO3��KI��������I2����ɲ���ƽ�������ӷ���ʽ��__IO3����____I����____===____I2��____H2O��
��2����Ӧ��1������I2��������_________________________��
��3���ζ��յ�ʱ��100mL��ˮ��������x mL����Һ��������1mL����Һ�൱��SO32��������1g�����ˮ����SO32���ĺ���Ϊ________mg��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ش��������⣺
��1���á�����������д�±���
��һ������ | �۵� | �е�(ͬѹ) | ���� |
P____S | MgO____CaS | CF4____SiCl4 | H��Cl____ H��Br |
��2��һ����ȡNH2OH�ķ�ӦΪ2NO2��+4SO2 + 6H2O+ 6NH3 = 4SO![]() +6NH4++2NH2OH��
+6NH4++2NH2OH��
��N��H��O�ĵ縺�Դ�С������˳��Ϊ____��
��NH3���ӵĿռ乹��Ϊ____��
��NH2OH��������H2O������Ϊ���Ƕ��Ǽ��Է����⣬����Ϊ____��
��3�������K[PtCl3(NH3)]��[PtCl3(NH3)]���Ľṹ����ʾ��ͼ��ʾΪ____�������ǿռ乹�ͣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��FeCl3ʴ��Һ��FeCl3���������ɣ�������ʴ��ͭ��ͭ�Ͻ�ȡ�
��1���������������Fe3+�����ɵ�Cu2+��ˮ�⣬ Fe3+ˮ������ӷ���ʽΪ____��
��2��FeCl3��Һʴ��ͭ�Ļ�ѧ����ʽΪ____��
��3���ⶨij��ʴ��Һ��Cu2+������ʵ�鲽�����£���ȡ25.00mL��ʴ��Һ������������NH4HF2���ڱ�Fe3+���������ţ��������Թ���KI��Һ��2Cu2++4I��=2CuI��+ I2�����ڰ�������5min��Ȼ����0.02000mol��L��1Na2S2O3����Һ�ζ���I2+2S2O![]() =2I��+S4O
=2I��+S4O![]() �����ӽ��յ�ʱ��������ۺ�10mL10%NH4SCN��Һ��CuI����������I2�� CuSCN������I2���������ζ����յ㣬����Na2S2O3��Һ20.00mL��
�����ӽ��յ�ʱ��������ۺ�10mL10%NH4SCN��Һ��CuI����������I2�� CuSCN������I2���������ζ����յ㣬����Na2S2O3��Һ20.00mL��
�ٲ���NH4HF2�ᵼ�²�õ�ͭ�ĺ���____�����ƫ�ߡ���ƫ�͡����䡱����
��ͨ������ȷ���÷�ʴ��Һ��ͭ�ĺ�������λg��L��1��д��������̣�____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��C��D��E��F��G��H��I�������ʣ�����A��B��D��E��ɫ��Ӧ��Ϊ��ɫ(����ɫ�ܲ���)��G��F�ǵ��ʣ������Ϊ�����H��һ�ֵ���ɫ���壬���ǵ�ת����ϵ��ͼ��ʾ����ش�
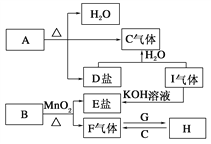
��1������G���ӽṹʾ��ͼ________��
��2��д��B��I�Ļ�ѧʽB________��I________��
��3��д��H��һ����;__________________________________��
��4��д��A���ȷֽ�Ļ�ѧ����ʽ_________________________��
��5����10g��C6H12O6�������г��ȼ�գ�������ȫ����������H��ַ�Ӧ����Ӧ���������____________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о�,�ܽ��������������H2�ķ�Ӧ:��Zn+����;��Na+ˮ;��Al+NaOH��Һ��Ϊ��ȼ����������Ӧ���ɵ�H2,�������������ͼ��ʾ��װ��ͼ:��ش���������:
![]()
��1��д��Al��NaOH��Һ��Ӧ�����ӷ���ʽ_______________________________��
��2���ڵ�ȼH2֮ǰ�����Ƚ���____________________________________________��

��3��ʵ��С���ڵ�ȼ������װ���Ƶõ�H2ʱ,�٢�ʵ���óɹ�,��ȴʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫��,Na��������̫�١����������������Ƶ�����,�ɽ�ʦ˵̫Σ��,����Ϊ����Σ�յ�ԭ����___________________________��
��4��ʵ��С������ơ���(һ�ֲ�����ˮ��Һ̬�л���)��ˮ���ܶȷֱ�Ϊ0.97 g��mL-1��0.88 g��mL-1��1.00 g��mL-1,���ݴ˶�ʵ������˸Ľ����ڸĽ����ʵ����H2����������____________��(���������������ӿ���)
��5����4.6gNa��8.1gAlͶ�뵽������ˮ�У�������Һ�����Ϊ200mL�������ɱ�״���µ�H2�������___________������Һ�����ʵ����ʵ���Ũ����__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��������������������أ�����˵���в���ȷ���ǣ� ��
A.�Ͻ�����п��ܺ��зǽ���Ԫ��
B.���ڳ�ʪ�Ŀ����з��ã�������ѧ��ʴ������
C.������(����)�ı���һ����������ʴ�ӿ�
D.����10�ŷɴ�����̫���ܵ�ذ�ɽ�����ת��Ϊ���ܣ�����ת�������ǵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Q��R��X��Y��Z����Ԫ�ص�ԭ���������ε�������֪����Z��ԭ������Ϊ29������ľ�Ϊ����������Ԫ�أ�Yԭ�ӵļ۵���(��Χ����)�Ų�Ϊmsnmpn����Rԭ�Ӻ���L�������Ϊ��������Q��Xԭ��p����ĵ������ֱ�Ϊ2��4����ش��������⣺
��1��Z2���ĺ�������Ų�ʽ��________��
��2����[Z(NH3)4]2�������У�Z2���Ŀչ������NH3�����ṩ��________�γ���λ����
��3��Q��Y�γɵ������̬�⻯��ֱ�Ϊ�ס��ң������ж���ȷ����________��
a���ȶ��ԣ���>�ң��е㣺��>�� b���ȶ��ԣ���>�ң��е㣺��<��
c���ȶ��ԣ���<�ң��е㣺��<�� d���ȶ��ԣ���<�ң��е㣺��>��
��4��Q��R��Y����Ԫ�صĵ�һ��������ֵ��С�����˳��Ϊ________(��Ԫ�ط�������)��
��5��Q��һ���⻯����Է�������Ϊ26�����з����е������������ļ���֮��Ϊ________��������ԭ�ӵ��ӻ�������________��
��6��ijԪ��ԭ�ӵļ۵��ӹ���Ϊ3d54s1����Ԫ������________��Ԫ�أ�Ԫ�ط�����________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com