
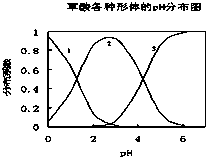
 ���ᣨH2C2O4����һ��������ˮ�Ķ�Ԫ��ǿ�ᣬ��ˮ�����Ĵ�����̬��H2C2O4��HC2O4-��C2O42-������̬�ķֲ�ϵ����Ũ�ȷ�����������ҺpH�仯�Ĺ�ϵ��ͼ��ʾ��
���ᣨH2C2O4����һ��������ˮ�Ķ�Ԫ��ǿ�ᣬ��ˮ�����Ĵ�����̬��H2C2O4��HC2O4-��C2O42-������̬�ķֲ�ϵ����Ũ�ȷ�����������ҺpH�仯�Ĺ�ϵ��ͼ��ʾ��| c(Na+) |
| c(C2O42-) |
| c(Na+) | ||
c(C2
|
| c(Na+) |
| c(C2O42-) |
| c(Na+) |
| c(C2O42-) |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ɱ������������ɱ��ƻ����� |
| B������ϩ��1��2-�������飻��������һ������ |
| C����ϩʹ��ˮ��ɫ����ϩʹ���Ը��������Һ��ɫ |
| D���ɱ����屽�����Ҵ����廯�ⷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ˮ����ˮ����ʳ�׳�ȥ��2H++CaCO3=Ca2++CO2��+H2O 2H++Mg��OH��2=Mg2++2H2O |
| B����NaHSO4��Һ�е���Ba��OH��2��Һ������H++SO42-+Ba2++OH-=BaSO4��+H2O |
| C����FeI2��Һ��ͨ������Cl2 2I-+Cl2=I2+2Cl- |
| D���������Ũ�ȵ�Ba��OH��2ϡ��Һ��NH4HCO3ϡ��Һ��ϣ�Ba2++2OH-+NH4++HCO3-=BaCO3��+NH3��+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
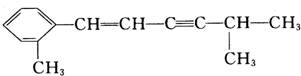
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
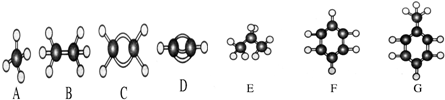
| �Լ� | ���� | |
| �������������ϩ | ||
| �屽���������嵥�� | ||
| ������������� | ||
| �Ҵ��л�������ˮ | ||
| ������л��������Ȼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com