
����Ŀ��ij�¶�ʱ��VIAԪ�ص�����H2��Ӧ������̬H2X���Ȼ�ѧ����ʽ���£�
![]() O2(g) +H2(g) ��H2O(g)
O2(g) +H2(g) ��H2O(g) ![]() H����242kJ��mol��1
H����242kJ��mol��1
S(g)+ H2(g) ��H2S(g) ![]() H����20kJ��mol��1
H����20kJ��mol��1
Se(g)+H2(g)![]() H2Se(g)
H2Se(g) ![]() H��+81kJ��mol��1
H��+81kJ��mol��1
����˵����ȷ����
A. �ȶ��ԣ�H2O< H2S< H2Se
B. ����������Se��H2��Ӧ����H2Se
C. O2(g)+2H2S(g)��2H2O(g)+2S(g) ![]() H����444 kJ��mol��1
H����444 kJ��mol��1
D. ���ź˵���������ӣ�VIA��Ԫ�ص�����H2�Ļ��Ϸ�ӦԽ������
���𰸡�C
��������
A�Ԫ�صķǽ�����Խǿ����Ӧ���⻯����ȶ���Խǿ��ͬһ���壬���ϵ��£��ǽ���������������ȶ��ԣ�H2O> H2S> H2Se����A�����
B�Se(g)+H2(g)![]() H2Se(g)��Ӧ����H>0��˵���÷�Ӧ�������ȣ�����ʹ�÷�Ӧ����ȵķ����ƶ����������ƶ�������������H2Se����B�����
H2Se(g)��Ӧ����H>0��˵���÷�Ӧ�������ȣ�����ʹ�÷�Ӧ����ȵķ����ƶ����������ƶ�������������H2Se����B�����
C�����֪�Ȼ�ѧ����ʽ���α��Ϊ���������ۣ����ݸ�˹���ɣ���2![]() (��-��)�ɵã�O2(g)+2H2S(g)��2H2O(g)+2S(g)
(��-��)�ɵã�O2(g)+2H2S(g)��2H2O(g)+2S(g) ![]() H����444 kJ��mol��1����C����ȷ��
H����444 kJ��mol��1����C����ȷ��
D����ź˵�����ļ�С����A��Ԫ�صķǽ���������ǿ������ԭ���γɵĹ��ۼ�Խǿ���ų�������Խ�࣬������Խ�ȶ�����A��Ԫ�صĵ�����H2�Ļ��Ϸ�ӦԽ����������D�����
����������������ȷ��ΪC��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ֽ�����ɵĺϽ�A���ں��ʵ������¿ɰ���ͼ���з�Ӧ(���ֲ����ˮʡ��)����֪FΪ���ɫ��������ش�
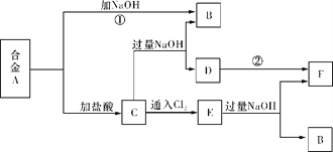
(1)��ҺC��������Ҫ��____________________(�ѧʽ)��
(2)д����Ӧ�ڵĻ�ѧ����ʽ_______________________________________________������D��F��ʵ������_______________________________________________��
(3)д����ӦC��E�����ӷ���ʽ________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�ᾧˮ���ﺬ��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() �е��������ӣ�ʵ��С��Ϊȷ���仯ѧʽ��������ʵ�飺
�е��������ӣ�ʵ��С��Ϊȷ���仯ѧʽ��������ʵ�飺
![]() ȷ��ȡ
ȷ��ȡ![]() ��Ʒ�����Ƴ�
��Ʒ�����Ƴ�![]() ��ҺX��
��ҺX��
![]() ȡ
ȡ![]() ��ҺX�������������ᣬ�����������ټ�����
��ҺX�������������ᣬ�����������ټ�����![]() ��Һ��������ɫ���������������ˡ�ϴ�ӡ����������أ��ð�ɫ����
��Һ��������ɫ���������������ˡ�ϴ�ӡ����������أ��ð�ɫ����![]() ��
��
![]() ȡ
ȡ![]() ��ҺX����������ϡ�����ữ����
��ҺX����������ϡ�����ữ����![]() ��Һ�ζ����յ㣬�ظ��ζ����Σ��������
��Һ�ζ����յ㣬�ظ��ζ����Σ��������![]() ��Һ�����ƽ��ֵΪ
��Һ�����ƽ��ֵΪ![]() ��
��
![]() �����ͼ��ʾ��װ�ã�ȡ
�����ͼ��ʾ��װ�ã�ȡ![]() ��Һ������ʵ�飬ʵ��ǰ��Bװ������
��Һ������ʵ�飬ʵ��ǰ��Bװ������![]() ��
��
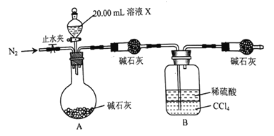
![]() ȡ������ҺX���μ�
ȡ������ҺX���μ�![]() ��Һ�����������������еμ�
��Һ�����������������еμ�![]() ��Һ���а�ɫ�������ɡ�
��Һ���а�ɫ�������ɡ�
�ش��������⣻
![]() ���ʵ��
���ʵ��![]() ����Ҫ�IJ��������У��ձ�������������Ͳ��____________��
����Ҫ�IJ��������У��ձ�������������Ͳ��____________��
![]() ʵ��
ʵ��![]() �ﵽ�ζ��յ��������_____________________��
�ﵽ�ζ��յ��������_____________________��
![]() ʵ��
ʵ��![]() �ķ�Ӧ������ֹˮ��ͨ��
�ķ�Ӧ������ֹˮ��ͨ��![]() ��������________________��
��������________________��
![]() ��������ʵ�����ݼ���ýᾧˮ����Ļ�ѧʽΪ______________��
��������ʵ�����ݼ���ýᾧˮ����Ļ�ѧʽΪ______________��
![]() ijͬѧ�������Ϸ��� AgSCNΪ��ɫ�����
ijͬѧ�������Ϸ��� AgSCNΪ��ɫ�����![]() ��������
��������![]() ��
��![]() ��Ϊ̽��
��Ϊ̽��![]() ��
��![]() �Ļ�ԭ��ǿ������ͬѧ�������ͼʵ��װ�ò���������ʵ�顣
�Ļ�ԭ��ǿ������ͬѧ�������ͼʵ��װ�ò���������ʵ�顣
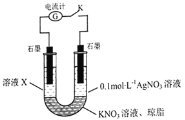
�ȶϿ����K������ҺX�еμ�![]() ��Һ������������˵��_____________���պϵ��K�����۲쵽��ʵ����������ҺX��졢�ұ�ʯī�缫���й���������������ָ��ƫת���ݴ˵ó��Ľ�����_____________����Һ����ԭ����___________
��Һ������������˵��_____________���պϵ��K�����۲쵽��ʵ����������ҺX��졢�ұ�ʯī�缫���й���������������ָ��ƫת���ݴ˵ó��Ľ�����_____________����Һ����ԭ����___________![]() �����ӷ���ʽ��ʾ
�����ӷ���ʽ��ʾ![]() ����ʵ����Ƶ��ŵ���_______________��
����ʵ����Ƶ��ŵ���_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪:��2H2(g)+O2(g)![]() 2H2O(l)����H=-571.6 kJ��mol-1
2H2O(l)����H=-571.6 kJ��mol-1
��2CH3OH(l)+3O2(g)![]() 2CO2(g)+4H2O(l)����H=-1 452 kJ��mol-1
2CO2(g)+4H2O(l)����H=-1 452 kJ��mol-1
��H+(aq)+OH-(aq)![]() H2O(l)����H=-57.3 kJ��mol-1
H2O(l)����H=-57.3 kJ��mol-1
����˵����ȷ����
A. H2(g)��ȼ����Ϊ142.9 kJ��mol-1
B. ͬ������H2(g)��CH3OH(l)��ȫȼ��,H2(g)�ų���������
C. 1/2H2SO4(aq)+1/2Ba(OH)2(aq)![]() 1/2BaSO4(s)+H2O(l)����H=-57.3 kJ��mol-1
1/2BaSO4(s)+H2O(l)����H=-57.3 kJ��mol-1
D. 3H2(g)+CO2(g)![]() CH3OH(l)+H2O(l)����H=+131.4 kJ��mol-1
CH3OH(l)+H2O(l)����H=+131.4 kJ��mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��Ӧ��![]() ʱ��
ʱ��![]() ��
��![]() ��
��![]() ϡ��Һ�У�
ϡ��Һ�У�![]() ��
��![]() ��
��![]() ���Ļ�ѧʽΪP�����Ļ�ѧʽΪ
���Ļ�ѧʽΪP�����Ļ�ѧʽΪ![]() ����֪��
����֪��
![]() ��
��![]()
![]() ��
��![]()
���н�����ȷ����
A.���ں���ת��Ϊ�����Ƿ��ȷ�Ӧ���������ĺ��������Ȱ���
B.ϡ�����ϡ����������Һ��Ӧ���к���![]()
C.̼��ȼ���ȴ���![]()
D.ϡ�����ϡ����������Һ��Ӧ����1molˮ���ų�![]() ����
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��W��Ϊ������Ԫ�أ�������Ԫ�����ڱ��е����λ����ͼ��ʾ����Zԭ�ӵ������������ǵ�һ���������3��������˵������ȷ����( )

A. X�������̬�⻯���ˮ��Һ������
B. ����������Ӧˮ���������W��Zǿ
C. Z�ĵ�����������Ӧ��Y������������Ӧ����
D. X��ԭ�Ӱ뾶С��Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о���������ķ�Ӧ����������������Ի�������Ⱦ����Ҫ���塣��ش������뵪Ԫ���йص�����:
(1)��������(�ṹʽΪCl-N=O)���л��ϳ��е���Ҫ�Լ���������Cl2��NO��ͨ�������·�Ӧ�Ƶã���Ӧ����ʽΪ2NO(g)+Cl2(g) ![]() 2ClNO(g)����֪���ֻ�ѧ�ļ����������±���ʾ:
2ClNO(g)����֪���ֻ�ѧ�ļ����������±���ʾ:

��Cl2��NO��Ӧ����ClNO�Ĺ�����ת����4mol���ӣ������Ϸų�������Ϊ____kJ.
(2)��һ�������ܱ������г���2molNO(g)��1 mol Cl2(g)����(1)�з�Ӧ�����¶ȷֱ�ΪT1��T2ʱ���NO�����ʵ���(��λ:mol)��ʱ��Ĺ�ϵ���±���ʾ
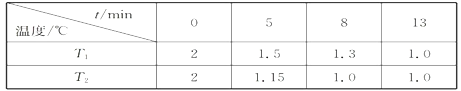
��T1________T2(�>������������)��
���¶�ΪT2��ʱ������ͬ�����У�����4molNO(g)��2mo1Cl2(g)����NO��ƽ��ת����___________50%(����ڡ��������ڡ���С�ڡ�)
���¶�ΪT2��ʱ����ʼʱ�����ڵ�ǿΪp0����÷�Ӧ��ƽ�ⳣ��Kp=______(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������)��
(3)������������ˮ�еĵ���Ⱦ�ѳ�Ϊһ�������ԵĻ������⡣�ڽ���Pt��Cu��ҿ(Ir)�Ĵ������£��ܱ������е�H2�ɸ�Чת��������Һ�е���̬��(NO3-)���乤��ԭ����ͼ��ʾ
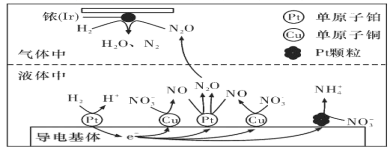
��Ir���淢����Ӧ�ķ���ʽΪ_________________________________________________��
������������ϵ�Pt�������࣬��ɵĺ����___________________________________��
II:���õ绯ѧԭ������NO2��O2������KNO3�Ƴ�ȼ�ϵ�أ�ģ�ҵ��ⷨ����������װ����ͼ��ʾ
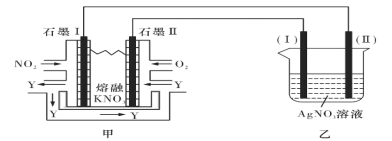
��ش���������:
(4)�ټ׳ع���ʱ��NO2ת�����ɫ������Y��Y��N2O5����ѭ��ʹ�ã���ʯīII���������ĵ缫��ӦʽΪ____________________________________________��
������10A�ĵ������60min����֪�õ��صĵ��Ч��Ϊ80.4���������������õ�____g������Ag��(����С�����һλ��ͨ��һ������ʱ������ʵ�ʳ����Ľ���������ͨ����ͬ����ʱ������Ӧ�����Ľ�������֮�Ƚе��Ч�ʡ������ڳ���Ϊ96500C/mol)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����2H2O(g)===2H2(g)��O2(g)����H����483.6 kJ��mol��1 ��H2S(g)===H2(g)��S(g)����H����20.1 kJ��mol��1�����ж���ȷ����(����)
A. ������ȼ���ȣ���H����241.8 kJ��mol��1
B. ��ͬ�����£����ȼ��1 mol H2(g)��1 mol S(g)�Ļ����ȳ��ȼ��1 mol H2S(g)���ȶ�20.1 kJ
C. �ɢ٢�֪��ˮ�����ȶ���С������
D. ���������ɹ�̬����H������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��C��D����Ԫ�ص�ԭ������֮�͵���36.A�ĵ�������������壻B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�С����������֮�ƣ��䵥�ʺͻ������й㷺����;��D4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ.��ҵ������DO2��̼�ᱵ������״̬����ȡ�������(�ɿ���һ�ֺ�������).��������������ġ�ѹ�����ܡ���Ӧ���ڳ������ķ���װ��.��X���߷��������������ľ����ṹΪ������(����ͼ��ʾ)������Ba2+ռ������λ�ã�O2-ռ������λ�ã�D4+ռ�ݶ���λ��.
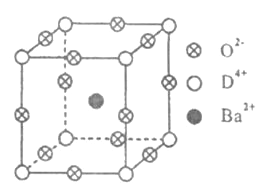
��ش��������⣺
��1��A��B��C����Ԫ�صĵ縺���ɴ�С��˳����__________________(��Ԫ�ط���).
��2��BA4���ӵĿռ乹����______________��Bԭ�ӹ�����ӻ�����Ϊ_____.
��3��C����̬�⻯��ĵ���ʽΪ____����е����ͬ��������Ԫ���⻯��ķе㣬��Ҫԭ����____________________.
��4��D�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ____________________.
��5�����Ʊ�������Ļ�ѧ����ʽΪ____________________.
���ڼ����У�����D4+��������������ģ�Ba2+����������Ķ��㣬��O2-�����������__________.
���ڼ����У�D4+������λ��Ϊ__________.
����֪�����Ħ������ΪM g/mol���侧���߳�Ϊ4.03��10-10m���������ܶ�Ϊ__________________g/cm3(Ҫ���г���ʽ�������ӵ�������NA��ʾ).
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com