
����Ŀ����(Se)�ǵ�34��Ԫ�أ��������ڲ��ɻ�ȱ����Ԫ�أ������γ�H2Se��SeO2��H2SeO3��H2SeO4��CuSe�ȶ��ֻ������ش��������⣺
��1������Ԫ�����ڱ��е�λ��________________________��
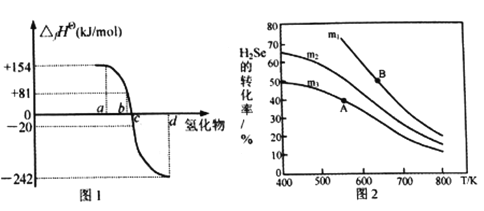
��2����101kPa��һ���¶�(һ����298K)�£����ȶ����ʷ�����Ӧ����1mol������ķ�Ӧ�Ƚиû�����ı�������(��fH��)��ͼ1Ϊ����Ԫ���⻯��a��b��c��d����̬ʱ�����������ݡ�
��ͼ1���⻯��d�ĵ���ʽΪ__________________________��
����298Kʱ��������ֽⷴӦ���Ȼ�ѧ��Ӧ����ʽΪ__________________________��
����ͼ�����ݼ��㣬2H2Se(g)+O2(g) ![]() 2Se(s)+2H2O(g) ��H=_____________KJ/mol
2Se(s)+2H2O(g) ��H=_____________KJ/mol
��3���ں��ݷ�Ӧ���У���H2Se(g)��O2(g)����ͬ����[n(H2Se)/n(O2)=m]Ͷ�뷴Ӧ������÷�Ӧ2H2Se(g)+O2(g) ![]() 2Se(s)+2H2O(g)��H2Se��ƽ��ת�������¶ȱ仯��ͼ2��ʾ����A��B����ƽ�ⳣ����С��ϵΪKA________KB(����<������>������=��)��ͼ��m1��m2��m3�ɴ�С��˳��Ϊ ____________��������____________________________________��
2Se(s)+2H2O(g)��H2Se��ƽ��ת�������¶ȱ仯��ͼ2��ʾ����A��B����ƽ�ⳣ����С��ϵΪKA________KB(����<������>������=��)��ͼ��m1��m2��m3�ɴ�С��˳��Ϊ ____________��������____________________________________��
��4���������ܶȻ���Ksp(CuSe)=7.9x10-49��Ksp(CuS)=1.3��10-36����ӦCuS(s)+Se2-(aq) ![]() CuSe(s)+S2-(aq)�Ļ�ѧƽ�ⳣ��KΪ____________(����ÿ�ѧ��������ʾ��������2λС��)������Һ��c(S2-)=100c(Se2-)ʱ����Ӧ��v(��)_____v����)(����<������>������=��) ��
CuSe(s)+S2-(aq)�Ļ�ѧƽ�ⳣ��KΪ____________(����ÿ�ѧ��������ʾ��������2λС��)������Һ��c(S2-)=100c(Se2-)ʱ����Ӧ��v(��)_____v����)(����<������>������=��) ��
���𰸡� �������ڢ�A�� ![]() H2Se(g)=H2(g)+Se(s)��H=-81kJ/mol -646 > m3>m2>m1 ��ͬ�¶��£�����O2(g)��Ũ�ȣ�mֵ��С��ƽ�������ƶ���H2Se��ƽ��ת�������� 1.65��1012 >
H2Se(g)=H2(g)+Se(s)��H=-81kJ/mol -646 > m3>m2>m1 ��ͬ�¶��£�����O2(g)��Ũ�ȣ�mֵ��С��ƽ�������ƶ���H2Se��ƽ��ת�������� 1.65��1012 >
����������1������Ԫ�����ڱ��е�λ��Ϊ������������A�壻
��2���ǽ���Ԫ���⻯����ȶ���������1mol�⻯��ʱ����H�Ĺ�ϵΪ������Ԫ�������ɣ�ͬһ����Ԫ�طǽ�����Խǿ��������̬�⻯��Խ���ף���̬�⻯��Խ�ȶ�������������ѧ������Խ��Խ�ȶ���a��b��c��d����ΪH2Te��H2Se��H2S��H2O��
��d�ĵ���ʽΪ![]() ��
��
����ͼ1��֪����298Kʱ�����������������81kJ/mol�����Էֽ�����H=-81kJ/mol���ʷֽⷴӦ���Ȼ�ѧ��Ӧ����ʽΪ��H2Se(g)=H2(g)+Se(s)��H=-81kJ/mol
����ͼ1��֪��H2O�������ȵ��ȷ���ʽΪH2+1/2O2=H2O ��H=-242kJ/mol��1����H2Se�ֽⷴӦ���Ȼ�ѧ��Ӧ����ʽΪ��H2Se(g)=H2(g)+Se(s)��H=-81kJ/mol��2�������ݸ�˹���ɣ���1����2+��2����2�ɵ�Ŀ�귽��ʽ������H=-242��2-81��2=-646 kJ/mol��
��3����ͼ2��֪������ת���ʽ��ͣ�������ƽ�ⳣ����С��B���¶ȸ���A�㣬��KA>KB����ͬ�¶��£�H2Se��ƽ��ת����m3<m2<m1�����n(H2Se)/n(O2)=m����ͬ�¶��£�����O2(g)��Ũ�ȣ�mֵ��С��ƽ�������ƶ���H2Se��ƽ��ת������������m3>m2>m1��
��4��������֪����ʽ���Եó���ѧƽ�ⳣ�� ������ѧƽ��ʱ��
������ѧƽ��ʱ�� ������ʱ��Һ��
������ʱ��Һ�� ��Ӧ��û��ƽ�Ⲣ��v(��)>v����)���ʴ�Ϊ��1.65��1012�� >��
��Ӧ��û��ƽ�Ⲣ��v(��)>v����)���ʴ�Ϊ��1.65��1012�� >��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ������У��ɴﵽ��Ӧʵ��Ŀ�ĵ���( )
ʵ����� | ʵ��Ŀ�� | |
A | ������ˮ��Ϻ�������� | ���屽 |
B | ij�л�����������Ȼ�̼��Һ��� | ȷ�ϸ��л��ﺬ̼̼˫�� |
C | �� | ������л����е���ԭ�� |
D | �Ҵ������Ը��������Һ��� | �����Ҵ����л�ԭ�� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����Ƽ��仯�����˵����ȷ���ǣ� ��
A.�Ƶ��ܶȱ�ˮ��B.�������ƿ��Ա�����ú����
C.��ȼ��ʱ����������D.�������Ż�������ˮ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ɡ����(ͼI)������Ⱦ�ϡ�ҽҩ�����ϵ��м��塣����˵���������
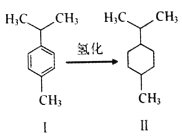
A. ���¶�ɡ������Һ̬��������ˮ
B. ͼ�����ʵ�һ�ȴ�����5�ֽṹ
C. ��ɡ���������9��̼ԭ�ӹ�ƽ��
D. ͼ���⻯��Ӧ���Ǽӳɷ�Ӧ���ǻ�ԭ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ö��Ե缫��ⷨ�Ʊ�����[H3BO3��B(OH) 3]�Ĺ���ԭ����ͼ��ʾ(��Ĥ����Ĥ�ֱ�ֻ���������Ӻ�������ͨ��)�������й�˵����ȷ���ǣ� ��
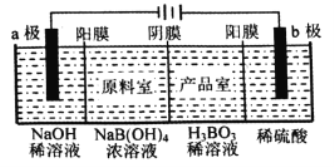
A. �������������������������Ϊ1��2 B. b���ĵ缫��ӦʽΪ2H2O��2e��=O2��+4H+
C. ��Ʒ���з����ķ�Ӧ��B(OH)3+OH��=B(OH)4�� D. ÿ����1 mol H3BO3��Ʒ��NaOH��Һ����22g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ҫ�ĺϽ�Ԫ�أ������������������ȺϽ���Ҳ�������л�����Ĵ�������ȡ����
��1��д����̬Cr�ļ����Ų�ʽ__________��Cr�й���__________�ֲ�ͬ�ܼ��ĵ��ӡ�
��2��Ni(CO)n��Fe(CO)5ͬ�������ʻ������γ������ʱ��ÿ��CO�ṩһ�Ե��������ԭ���γ���λ�����о����ֽ���ԭ�ӵļ۵��Ӻ�CO�ṩ�ĵ����ܺ͵���18��
��Ni��C��O�ĵ縺���ɴ�С��˳��Ϊ____________________��
�� Ni(CO)n������n=__________��
����֪Ni2+��Fe2+�����Ӱ뾶�ֱ�Ϊ69pm��78pm�������ҽ������������ڵ�NiO��FeO�ҽ�����ȴ�����У�NiO�������Ƚᾧ���Խ�����ԭ��____________________��
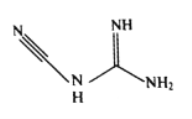
��3���춨�����ܡ�ͭ�ٵȿ���˫�谷����ѧʽC2H4N4����ṹ��ʽ��ͼ��ʾ��˫�谷������̼ԭ�ӵ��ӻ���ʽ��__________�����ӽṹ�м������Ĺ��ۼ���__________��
��4�����ľ���ṹ�������Ͻ�ľ�����ͼ��ʾ��
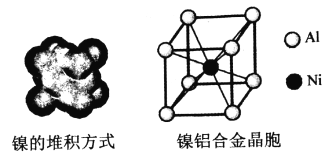
��������Ķѻ���ʽΪ__________��
����֪Al�ĵ�һ���ڶ������ֱܷ�Ϊ��l1=578kJ/mol��l2=1817kJ/mol������l2Զ����l1��ԭ��__________________________________________________��
����֪�������Ͻ��ܶ�Ϊdg/cm3��NA��������٤���������������ĺ˼��Ϊ__________ pm��(�ô���ʽ��ʾ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2018��ֵ����������������г����淢�ȸ��յ�֢״���������һ�ֳ��õĶ�ͯ����ҩ������BHC�ϳɷ�����
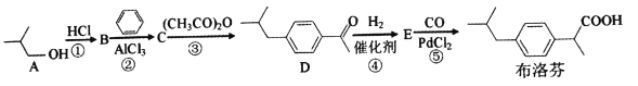
��1��A��������__________��B�ĺ˴Ź���������__________�����շ塣
��2������Ӧ����Ũ���������ϡ���ᣬ��ԭ����______________________________��
��3��E�ķ���ʽ��__________�������ŵ�������__________��
��4������Ӧ�Ļ�ѧ����ʽ��____________________����Ӧ������__________��
��5���������������IJ���ҵ�ͬ���칹����__________��
a��FeCl3��Һ����ɫ b.��������5�ֲ�ͬ��ѧ�������� c.�ܷ���������Ӧ
��6����д���ɱ���( CH3COOCH2CO)2OΪԭ�Ϻϳ�![]() ����·ͼ(�����Լ���ѡ)___________
����·ͼ(�����Լ���ѡ)___________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Լ������ڳ�ȥ���������е���ϩ�������
A.��ˮB.������Ȼ�̼��ҺC.���Ը��������ҺD.����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������G��һ��ҽҩ�м��壬�ɷ��㻯����A�Ʊ�G��һ�ֺϳ�·�����£�

�ش��������⣺��֪�������ܱ�LiAlH4��ԭΪ��
��
(1)A�Ļ�ѧ����_________________��
(2)C�Ľṹ��ʽ_______________����B����C�ķ�Ӧ����_____________��
(3)��F����G�ķ�Ӧ����ʽ_____________________��
(4)���㻯����X��C��ͬ���칹�壬1mol X������̼��������Һ��Ӧ����88g CO2����˴Ź���������ʾ��3�ֲ�ͬ��ѧ�������⣬�����֮��Ϊ3��1��1����________�֣�д��1�ַ���Ҫ���X�Ľṹ��ʽ__________________��
(5)���������ϳ�·�ߣ�д���ü״��ͱ��״�Ϊԭ���Ʊ������� �ĺϳ�·��__________��
�ĺϳ�·��__________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com