
ЎҫМвДҝЎҝIЎўДіС§ЙъУГ0.2000 molЎӨLЈӯ1өДұкЧјNaOHИЬТәөО¶ЁОҙЦӘЕЁ¶ИөДСОЛбЈ¬ЖдІЩЧчҝЙ·ЦОӘИзПВјёІҪЈә
ўЩУГХфБуЛ®ПҙөУјоКҪөО¶Ё№ЬЈ¬ІўЧўИлNaOHИЬТәЦБЎ°0ЎұҝМ¶ИПЯТФЙП
ўЪ№М¶ЁәГөО¶Ё№ЬІўК№өО¶Ё№ЬјвЧмідВъТәМе
ўЫөчҪЪТәГжЦБЎ°0Ўұ»тЎ°0ЎұҝМ¶ИПЯЙФПВЈ¬ІўјЗПВ¶БКэ
ўЬБҝИЎ20.00mLҙэІвТәЧўИлИуПҙ№эөДЧ¶РОЖҝЦРЈ¬ІўјУИл1»т2өО·УМӘИЬТә
ўЭөОИлТ»өОұкЧјТәәуЈ¬ИЬТәСХЙ«УЙОЮЙ«ұдОӘәмЙ«БўјҙНЈЦ№өО¶ЁЈ¬јЗВјТәГж¶БКэ
Зл»ШҙрЈә

ЈЁ1Ј©ТФЙПІҪЦиУРҙнОуөДКЗЈЁМоұаәЕЈ©________ЎЈ
ЈЁ2Ј©УГұкЧјNaOHИЬТәөО¶ЁКұЈ¬УҰҪ«ұкЧјNaOHИЬТәЧўИл______ЦРЎЈЈЁҙУНјЦРСЎМоЎ°јЧЎұ»тЎ°ТТЎұЈ©
ЈЁ3Ј©ПВБРІЩЧч»бТэЖрКөСйҪб№ыЖ«ҙуөДКЗЈә______ЈЁМоұаәЕЈ©
A ЛбКҪөО¶Ё№ЬОҙИуПҙ
B өО¶ЁЗ°Ј¬өО¶Ё№ЬјвЧмОЮЖшЕЭЈ¬өО¶ЁәуУРЖшЕЭ
C Ч¶РОЖҝПИУГХфБуЛ®ПҙөУәуЈ¬ОҙУГҙэІвТәИуПҙ
D өО¶ЁҪбКшКұСцКУөО¶Ё№ЬЈ¬ІўјЗВјКэҫЭ
E өО¶Ё№эіМЦРУРТ»өОұкЧјТә·ЙҪҰіцЧ¶РОЖҝ
ЈЁ4Ј©өО¶ЁКұЈ¬ЧуКЦҝШЦЖөО¶Ё№ЬЈ¬УТКЦТЎ¶ҜЧ¶РОЖҝЈ¬СЫҫҰЧўКУ_______________ЎЈ
II.АыУГЦРәНөО¶ЁөДФӯАнЈ¬ФЪ№ӨТөЙъІъЦР»№ҝЙТФҪшРРСх»Ҝ»№ФӯөО¶ЁІв¶ЁОпЦКә¬БҝЎЈ
ЈЁ5Ј©Л®ДаЦРёЖҫӯҙҰАнөГІЭЛбёЖіБөнҫӯПЎH2SO4ҙҰАнәу,УГ![]() ұкЧјИЬТәөО¶Ё,НЁ№эІв¶ЁІЭЛбөДБҝҝЙјдҪУ»сЦӘёЖөДә¬Бҝ,өО¶Ё·ҙОӘ:
ұкЧјИЬТәөО¶Ё,НЁ№эІв¶ЁІЭЛбөДБҝҝЙјдҪУ»сЦӘёЖөДә¬Бҝ,өО¶Ё·ҙОӘ:![]() .КөСйЦРіЖИЎ0.400gЛ®ДаСщЖ·,өО¶ЁКұПыәДБЛ0.0500 molЎӨLЈӯ1өД
.КөСйЦРіЖИЎ0.400gЛ®ДаСщЖ·,өО¶ЁКұПыәДБЛ0.0500 molЎӨLЈӯ1өД![]() ИЬТә36.00 mL,ФтёГЛ®ДаСщЖ·ЦРёЖөДЦКБҝ·ЦКэОӘ__________
ИЬТә36.00 mL,ФтёГЛ®ДаСщЖ·ЦРёЖөДЦКБҝ·ЦКэОӘ__________
ЈЁ6Ј©өО¶ЁЦХөгөДПЦПуКЗ__________ЎЈ
Ўҫҙр°ёЎҝўЩўЬўЭ ТТ DЎўE Ч¶РОЖҝДЪИЬТәСХЙ«ұд»Ҝ 45.0©Ү өОИлЧоәуТ»өОұкЧјТәёЯГМЛбјШәуЈ¬Ч¶РОЖҝДЪИЬТәЗЎәГУЙОЮЙ«ұдОӘЈЁЗіЈ©ЧПЙ«Ј¬ЗТ°л·ЦЦУІ»»ЦёҙФӯАҙСХЙ«
ЎҫҪвОцЎҝ
ЈЁ1Ј©ўЩөО¶Ё№ЬУГХфБуЛ®ПҙНкәуЈ¬ұШРлИуПҙЈ¬І»ИуПҙөО¶Ё№ЬЈ¬»бК№ұкЧјТәөДЕЁ¶ИјхРЎЈ»
ЈЁ2Ј©јЧКЗЛбКҪөО¶Ё№ЬЈ¬ТТКЗјоКҪөО¶Ё№ЬЈ»
ЈЁ3Ј©AЈ®Ч¶РОЖҝУРЛ®Ј¬І»У°ПмөО¶ЁҪб№ыЈ»
BЈ®јоКҪөО¶Ё№ЬјвЧмУРЖшЕЭЈ¬ПыәДөДұкЧјТәФцҙуЈ¬Ҫб№ыЖ«ёЯЈ»
CЈ®Ч¶РОЖҝПИУГХфБуЛ®ПҙөУәуЈ¬ОҙУГҙэІвТәИуПҙЈ¬ОЮУ°ПмЈ»
DЈ®УГЛбКҪөО¶Ё№ЬБҝИЎТәМеКұЈ¬КН·ЕТәМеЗ°өО¶Ё№ЬЗ°¶ЛУРЖшЕЭЈ¬Ц®әуПыК§Ј¬ҙэІвТәЖ«РЎЈ¬ЕЁ¶ИЖ«өНЈ»
ЈЁ4Ј©өО¶ЁөҪИЬТәУРОЮЙ«ұдіЙЗіәмЙ«КұЈ¬ЗТ°л·ЦЦУЦ®ДЪІ»ФЩёДұдЈ¬өҪҙпөО¶ЁЦХөгЈ»
ЈЁ5Ј©ёщҫЭ№ШПөКҪ5Ca2+Ў«5H2C2O4Ў«2KMnO4ҪшРРјЖЛгЈ»
ЈЁ6Ј©өО¶ЁЦХөгёЯГМЛбјШЙФ№эБҝПФЧПЙ«Ј¬ФтИЬТәУЙОЮЙ«ұдОӘЈЁЗіЈ©ЧПЙ«ЎЈ
ЈЁ1Ј©ўЩөО¶Ё№ЬУГХфБуЛ®ПҙәуЈ¬І»ИуПҙЈ¬К№ұкЧјТәөДЕЁ¶ИјхРЎЈ¬ПыәДөДМе»эФцҙуЈ¬Ів¶ЁҪб№ыЖ«ҙуЈ¬ЛщТФұШРлУГЗвСх»ҜДЖИЬТәИуПҙЈ¬№КўЩІЩЧчУРОуЈ»
ўЬИфУГҙэІвТәИуПҙЧ¶РОЖҝЈ¬»бК№ҙэІвТәИЬЦКОпЦКөДБҝФцјУЈ¬ПыәДұкЧјТәөДМе»эФцҙуЈ¬Ів¶ЁҪб№ыЖ«ҙуЈ¬ЛщТФЧ¶РОЖҝІ»ДЬИуПҙЈ¬№КўЬІЩЧчУРОуЈ»
ўЭөОИлТ»өОұкЧјТәәуЈ¬ИЬТәСХЙ«УЙОЮЙ«ұдОӘәмЙ«БўјҙНЈЦ№өО¶ЁЈ¬ҙЛКұОҙҙпЦХөгЈ¬УҰКЗОЮЙ«ұдОӘәмЙ«ЗТ°л·ЦЦУДЪІ»ФЩёДұдЈ¬өО¶ЁҪбКшЈ¬№КўЭІЩЧчУРОуЈ»
№Кҙр°ёОӘЈәўЩўЬўЭЈ»
ЈЁ2Ј©ЗвСх»ҜДЖУҰёГУГјоКҪөО¶Ё№ЬЈ¬№КСЎТТЈ»
ЈЁ3Ј©ёщҫЭcЈЁҙэІвЈ©=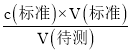 ·ЦОцІ»өұІЩЧч¶ФVЈЁұкЧјЈ©өДУ°ПмЈә
·ЦОцІ»өұІЩЧч¶ФVЈЁұкЧјЈ©өДУ°ПмЈә
AЈ®ЛбКҪөО¶Ё№ЬОҙИуПҙЈ¬ҙэІвТәұ»ПЎКНЈ¬ЕЁ¶ИұдРЎЈ¬ПыәДұкЧјТәөДМе»эјхРЎЈ¬К№өО¶ЁҪб№ыЖ«РЎЈ¬СЎПоAІ»·ыЈ»
BЈ®өО¶ЁЗ°Ј¬өО¶Ё№ЬјвЧмОЮЖшЕЭЈ¬өО¶ЁәуУРЖшЕЭЈ¬ПыәДөДұкЧјТәөДМе»эјхРЎЈ¬К№өО¶ЁҪб№ыЖ«РЎЈ¬СЎПоBІ»·ыЈ»
CЈ®Ч¶РОЖҝПИУГХфБуЛ®ПҙөУәуЈ¬ОҙУГҙэІвТәИуПҙЈ¬І»У°ПмөО¶ЁҪб№ыЈ¬СЎПоCІ»·ыЈ»
DЈ®өО¶ЁҪбКшКұСцКУөО¶Ё№ЬЈ¬ІўјЗВјКэҫЭЈ¬К№ұкЧјТәөДМе»эЖ«ҙуЈ¬К№өО¶ЁҪб№ыЖ«ҙуЈ¬СЎПоDҝЙСЎЈ»
EЈ®өО¶Ё№эіМЦРУРТ»өОұкЧјТә·ЙҪҰіцЧ¶РОЖҝЈ¬ПыәДөДұкЧјТәөДМе»эФцҙуЈ¬К№өО¶ЁҪб№ыЖ«ҙуЈ¬СЎПоEҝЙСЎЈ»
ҙр°ёСЎDEЈ»
ЈЁ4Ј©өО¶ЁКұЈ¬ЧуКЦҝШЦЖөО¶Ё№ЬЈ¬УТКЦТЎ¶ҜЧ¶РОЖҝЈ¬СЫҫҰЧўКУЧ¶РОЖҝДЪИЬТәСХЙ«ұд»ҜЈ¬
№Кҙр°ёОӘЈәЧ¶РОЖҝДЪИЬТәСХЙ«ұд»ҜЈ»
ЈЁ5Ј©·ҙУҰөД№ШПөКҪОӘ5Ca2+Ў«5H2C2O4Ў«2KMnO4Ј¬
n(KMnO4)=0.0500mol/LЎБ36.00mL=1.80mmolЈ¬
n(Ca2+)=4.50mmolЈ¬
Л®ДаЦРёЖөДЦКБҝ·ЦКэОӘ![]() ЎБ100%=45.0%Ј»
ЎБ100%=45.0%Ј»
ЈЁ6Ј©өО¶ЁЦХөгөДПЦПуКЗөОИлЧоәуТ»өОұкЧјТәёЯГМЛбјШәуЈ¬Ч¶РОЖҝДЪИЬТәЗЎәГУЙОЮЙ«ұдОӘЈЁЗіЈ©ЧПЙ«Ј¬ЗТ°л·ЦЦУІ»»ЦёҙФӯАҙСХЙ«ЎЈ


 С§Б·ҝміөөАҝмАЦјЩЖЪКојЩЧчТөРВҪ®ИЛГсіц°жЙзПөБРҙр°ё
С§Б·ҝміөөАҝмАЦјЩЖЪКојЩЧчТөРВҪ®ИЛГсіц°жЙзПөБРҙр°ё ХгҙуУЕѧСѧДкј¶ПОҪУөјУлБ·ХгҪӯҙуС§іц°жЙзПөБРҙр°ё
ХгҙуУЕѧСѧДкј¶ПОҪУөјУлБ·ХгҪӯҙуС§іц°жЙзПөБРҙр°ё РЎС§КојЩЧчТө¶«ДПҙуС§іц°жЙзПөБРҙр°ё
РЎС§КојЩЧчТө¶«ДПҙуС§іц°жЙзПөБРҙр°ё ҪтЗЕҪМУэКојЩ°ОёЯПОҪӹ㶫ИЛГсіц°жЙзПөБРҙр°ё
ҪтЗЕҪМУэКојЩ°ОёЯПОҪӹ㶫ИЛГсіц°жЙзПөБРҙр°ё ІЁІЁРЬКојЩЧчТөҪӯОчИЛГсіц°жЙзПөБРҙр°ё
ІЁІЁРЬКојЩЧчТөҪӯОчИЛГсіц°жЙзПөБРҙр°ё
| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝҙУЖПМСЧСЦРМбИЎөДФӯ»ЁЗаЛШҪб№№ИзНјЈ¬ҫЯУРЙъОп»оРФЈ¬Изҝ№Сх»ҜәНЧФУЙ»щЗеіэДЬБҰөИЎЈУР№ШФӯ»ЁЗаЛШөДПВБРЛө·ЁІ»ХэИ·өДКЗЈЁ Ј©
A.ёГОпЦКҝЙТФҝҙЧчҙјАаЈ¬ТІҝЙҝҙЧц·УАа
B.1molёГОпЦКҝЙУл7molNa2CO3·ҙУҰ
C.ёГОпЦКУцFeCl3»б·ўЙъПФЙ«·ҙУҰ
D.1molёГОпЦКҝЙУл4molBr2·ҙУҰ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝөх°Чҝй( NaHSO2ЎӨHCHOЎӨ2H2OЈ¬M=154.0g/mol)ФЪ№ӨТөЦРУР№г·әУҰУГЈ»өх°ЧҝйФЪЛбРФ»·ҫіПВЎў100Ўжјҙ·ўЙъ·ЦҪвКН·ЕіцHCHOЎЈКөСйКТЦЖұёөх°ЧҝйөД·Ҫ°ёИзПВЈә
NaHSO3өДЦЖұёЈә
ИзНјЈ¬ФЪ№гҝЪЖҝЦРјУИлТ»¶ЁБҝNa2SO3әНЛ®Ј¬ХсөҙИЬҪвЈ¬»әВэНЁИлSO2Ј¬ЦБ№гҝЪЖҝЦРИЬТәpHФјОӘ4Ј¬ЦЖөГNaHSO3ИЬТәЎЈ
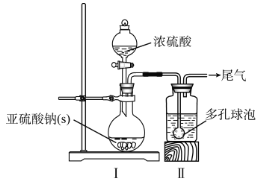
ЈЁ1Ј©Ч°ЦГўсЦРІъЙъЖшМеөД»ҜС§·ҙУҰ·ҪіМКҪОӘ__Ј»ўтЦР¶аҝЧЗтЕЭөДЧчУГКЗ__ЎЈ
ЈЁ2Ј©КөСйКТјмІвNaHSO3ҫ§МеФЪҝХЖшЦРКЗ·с·ўЙъСх»ҜұдЦКөДКөСй·Ҫ°ёКЗ__ЎЈ
өх°ЧҝйөДЦЖұёЈә
ИзНјЈ¬ПтТЗЖчAЦРјУИлЙПКцNaHSO3ИЬТәЎўЙФ№эБҝөДРҝ·ЫәНТ»¶ЁБҝјЧИ©Ј¬ФЪ80~90ЎжCПВЈ¬·ҙУҰФј3hЈ¬АдИҙ№эВЛЎЈ

ЈЁ3Ј©ТЗЖчAөДГыіЖОӘ___Ј»УГәгС№В©¶·ҙъМжЖХНЁөОТәВ©¶·өОјУјЧИ©өДУЕөгКЗ__ЎЈ
ЈЁ4Ј©Ҫ«ТЗЖчAЦРөД·ҙУҰОВ¶Иәг¶ЁФЪ80~90ЎжөДДҝөДКЗ__ЎЈ
өх°Чҝйҙҝ¶ИөДІв¶ЁЈә
Ҫ«0.5000gөх°ЧҝйСщЖ·ЦГУЪХфБуЙХЖҝЦРЈ¬јУИл10%БЧЛб10mLЈ¬БўјҙНЁИл100ЎжЛ®ХфЖшЈ»өх°Чҝй·ЦҪвІўКН·ЕіцјЧИ©Ј¬УГә¬36.00mL0.1000molЎӨLЈӯ1ЛбРФKMnO4ОьКХјЧИ©(І»ҝјВЗSO2У°ПмЈ¬4MnO4Јӯ+5HCHO+12H+=4Mn2++5CO2Ўь+11H2O)Ј¬ФЩУГ0.1000molЎӨLЈӯ1өДІЭЛбұкЧјИЬТәөО¶ЁЛбРФKMnO4Ј¬ФЩЦШёҙКөСй2ҙОЈ¬ЖҪҫщПыәДІЭЛбИЬТәөДМе»эОӘ30.00mLЎЈ
ЈЁ5Ј©өО¶ЁЦХөгөДЕР¶П·Ҫ·ЁКЗ__Ј»өх°ЧҝйСщЖ·өДҙҝ¶ИОӘ__%(ұЈБфЛДО»УРР§КэЧЦ)Ј»ИфKMnO4ұкЧјИЬТәҫГЦГКН·ЕіцO2¶шұдЦКЈ¬»бөјЦВІвБҝҪб№ы__(МоЎ°Ж«ёЯЎұЎўЎ°Ж«өНЎұ»тЎ°ОЮУ°ПмЎұ)
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝФЪ2LГЬұХИЭЖчДЪЈ¬јУИл0.100molCOЖшМеәН0.080molCuO№ММеЈ¬800ЎжКұ·ўЙъИзПВ·ҙУҰЈә2CuOЈЁsЈ©+COЈЁgЈ©![]() Cu2OЈЁsЈ©+CO2ЈЁgЈ©Ј¬nЈЁCuOЈ©ЛжКұјдөДұд»ҜИзұнЈә
Cu2OЈЁsЈ©+CO2ЈЁgЈ©Ј¬nЈЁCuOЈ©ЛжКұјдөДұд»ҜИзұнЈә
КұјдЈЁminЈ© | 0 | 1 | 2 | 3 | 4 | 5 |
nЈЁCuOЈ©ЈЁmolЈ© | 0.080 | 0.060 | 0.040 | 0.020 | 0.020 | 0.020 |
ЈЁ1Ј©УГCOұнКҫЗ°2minДЪөД»ҜС§·ҙУҰЛЩВК=_______
ЈЁ2Ј©јЖЛгҙЛ·ҙУҰФЪ800CКұөД»ҜС§ЖҪәвіЈКэK=_______
ЈЁ3Ј©ИфПтЖҪәвәуөДМеПөЦРјУИлCOәНCO2ёч0.05molЈ¬ФтҙЛКұVЈЁХэЈ©_______VЈЁДжЈ©
ЈЁ4Ј©УГАҙ»№ФӯCuOөДCOҝЙТФУГCәНЛ®ХфЖш·ҙУҰЦЖөГЎЈ
ТСЦӘЈәCЈЁsЈ©Ј«O 2ЈЁgЈ©= CO2ЈЁgЈ© H=-393.5kJ/mol
2CO(g)+O2(g)=2CO2(g) H=-566kJ/mol
2H2(g)+O2(g)=2H2O(g) H=-571.6kJ/mol
ФтCЈЁsЈ©Ј«H2OЈЁgЈ©![]() COЈЁgЈ©Ј«H2ЈЁgЈ© H= __________ЎЈ
COЈЁgЈ©Ј«H2ЈЁgЈ© H= __________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝКөСйКТҙУә¬өв·ПТә![]() іэ
іэ![]() НвЈ¬ә¬УР
НвЈ¬ә¬УР![]() Ўў
Ўў![]() Ўў
Ўў![]() өИ
өИ![]() ЦР»ШКХөвЈ¬ЖдКөСй№эіМИзПВЈә
ЦР»ШКХөвЈ¬ЖдКөСй№эіМИзПВЈә
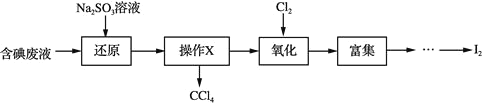
ПВБРРрКцІ»ХэИ·өДКЗ
A.Ў°»№ФӯЎұІҪЦи·ўЙъөД·ҙУҰОӘЈә![]()
![]()
B.Ў°ІЩЧчXЎұОӘҫІЦГЎў·ЦТәЈ¬ЛщөГ![]() ҝЙУГЧчЎ°ё»јҜЎұөДЭНИЎјБ
ҝЙУГЧчЎ°ё»јҜЎұөДЭНИЎјБ
C.Ў°Сх»ҜЎұ№эіМЦРЈ¬ОӘК№![]() НкИ«ұ»Сх»ҜЈ¬РиіӨКұјдНЁИл
НкИ«ұ»Сх»ҜЈ¬РиіӨКұјдНЁИл![]()
D.Ў°ё»јҜЎұјҙ![]() ё»јҜУЪУР»ъИЬјБЈ¬Н¬КұіэИҘДіР©ФУЦКАлЧУ
ё»јҜУЪУР»ъИЬјБЈ¬Н¬КұіэИҘДіР©ФУЦКАлЧУ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝQЎўXЎўYЎўZЎўWОеЦЦФӘЛШөДФӯЧУРтКэТАҙОөЭФцЈ¬WОӘөЪЛДЦЬЖЪФӘЛШЈ¬ЖдУаҫщОӘ¶МЦЬЖЪЦчЧеФӘЛШЎЈТСЦӘЈә
ўЩQФӯЧУөДЧоНвІгөзЧУКэКЗҙОНвІгөзЧУКэөД2ұ¶Ј»
ўЪYЎўZН¬ЦчЧеЈ¬YФӯЧУјЫөзЧУЕЕІјНјОӘ![]()
ўЫWФӘЛШ»щМ¬ФӯЧУөДMІгИ«ідВъЈ¬NІгГ»УРіЙ¶ФөзЧУЈ¬Ц»УРТ»ёцОҙіЙ¶ФөзЧУЎЈ
Зл»ШҙрПВБРОКМвЈә
(1)WөДФӘЛШГыіЖОӘ________Ј¬Жд»щМ¬ФӯЧУөДөзЧУЕЕІјКҪОӘ_________ЎЈ
(2)ҫЯУРПаН¬јЫөзЧУКэәНПаН¬ФӯЧУКэөД·ЦЧУ»тАлЧУҫЯУРПаН¬өДҪб№№МШХчЈ¬ХвТ»ФӯАніЖОӘЎ°өИөзЧУФӯАнЎұЈ¬ОеЦЦФӘЛШЦРөзёәРФЧоЗҝөД·ЗҪрКфФӘЛШРОіЙөДТ»ЦЦөҘЦКAУлYЎўZРОіЙөД»ҜәПОпBКЗөИөзЧУМеОпЦКЈ¬AЎўB·ЦЧУКҪ·ЦұрОӘ____________Ўў____________ЎЈ
(3)QЎўXЎўYИэЦЦФӘЛШөДөЪТ»өзАлДЬЧоҙуөДКЗ_______(МоФӘЛШ·ыәЕ)ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝ№ӨТөЙПЦЖИЎCuCl2өДЙъІъБчіМИзПВЈә
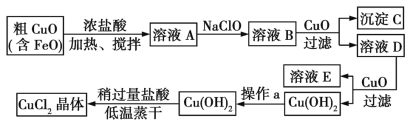
ЗлҪбәППВұнКэҫЭЈ¬»ШҙрОКМвЈә
ОпЦК | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
ИЬ¶И»э(25 Ўж) | 8.0ЎБ10-16 | 2.2ЎБ10-20 | 4.0ЎБ10-38 |
НкИ«іБөнКұөДpH·¶О§ | ЎЭ9.6 | ЎЭ6.4 | 3~4 |
(1)ФЪИЬТәAЦРјУИлNaClOөДДҝөДКЗ_________________ЎЈ
(2)ФЪИЬТәBЦРјУИлCuOЦчТӘЙжј°өДАлЧУ·ҙУҰ·ҪіМКҪОӘ________________ЎЈ
(3)ІЩЧчaОӘ___________ЎЈ
(4)ФЪCu(OH)2ЦРјУИлСОЛбК№Cu(OH)2ЧӘ»ҜОӘCuCl2Ј¬ІЙУГЙФ№эБҝСОЛбәНөНОВХфёЙөДДҝөДКЗ___ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝ·ҙУҰ2NO2ЈЁgЈ©![]() N2 O4ЈЁgЈ©Ј»ЎчH= Јӯ57 kJЎӨmolЈӯ1Ј¬ФЪОВ¶ИОӘT1ЎўT2КұЈ¬ЖҪәвМеПөЦРNO2өДМе»э·ЦКэЛжС№Зҝұд»ҜЗъПЯИзНјЛщКҫЎЈПВБРЛө·ЁХэИ·өДКЗ
N2 O4ЈЁgЈ©Ј»ЎчH= Јӯ57 kJЎӨmolЈӯ1Ј¬ФЪОВ¶ИОӘT1ЎўT2КұЈ¬ЖҪәвМеПөЦРNO2өДМе»э·ЦКэЛжС№Зҝұд»ҜЗъПЯИзНјЛщКҫЎЈПВБРЛө·ЁХэИ·өДКЗ

AЈ®AЎўCБҪөгөД·ҙУҰЛЩВКЈәA>C
BЈ®AЎўCБҪөгЖшМеөДСХЙ«ЈәAЗіЈ¬CЙо
CЈ®УЙЧҙМ¬AөҪЧҙМ¬BЈ¬ҝЙТФУГјУИИөД·Ҫ·Ё
DЈ®AЎўCБҪөгЖшМеөДЖҪҫщПа¶Ф·ЦЧУЦКБҝЈәA>C
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝТ»Сх»Ҝ¶юВИЈЁCl2OЈ©КЗТ»ЦЦіЈУГөДВИ»ҜјБЎЈіЈОВПВЈ¬Cl2OКЗЧШ»ЖЙ«ЎўУРҙМјӨРФЖшО¶өДЖшМеЈ¬ИЫ өгОӘ-120.6ЎгC,·РөгОӘ2.0ЎгC,ТЧУлЛ®·ҙУҰЙъіЙҙОВИЛбЎЈКөСйКТУыУГВИЖшНЁИлә¬Л®8%өДМјЛбДЖ№ММеЦР ЦЖұёІўКХјҜЙЩБҝҙҝҫ»өДCl2O,ЗлУГПВБРЧ°ЦГЙијЖКөСйІўМҪҫҝПа№ШОпЦКөДРФЦКЎЈ
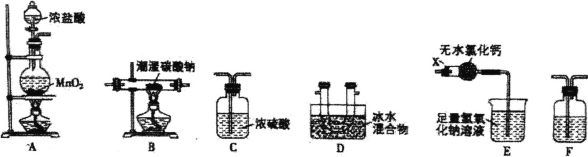
ЈЁ1Ј©Ч°ЦГEЦРТЗЖчXөДГыіЖОӘ ______ЎЈ
ЈЁ2Ј©Ч°ЦГөДБ¬ҪУЛіРтКЗA __________ЈЁГҝёцЧ°ЦГПЮУГТ»ҙОЈ©ЎЈ
ЈЁ3Ј©Ч°ЦГFЦРКўЧ°КФјБөДГыіЖОӘ______Ј¬Ч°ЦГEЦРОЮЛ®ВИ»ҜёЖөДЧчУГКЗ ________.ЎЈ
ЈЁ4Ј©Ч°ЦГBІРБф№ММеЦРіэNaClНв»№ә¬УРТ»ЦЦЛбКҪСОM,РҙіцЧ°ЦГBЦР·ўЙъ·ҙУҰөД»ҜС§·ҪіМКҪ _______ЎЈ
ЈЁ5Ј©ЦӨГчІРБф№ММеЦРә¬УРMөДЧојтөҘөДКөСй·Ҫ°ёКЗ: _______ЎЈ
ЈЁ6Ј©Ів¶ЁІРБф№ММеЦРMөДЦКБҝ·ЦКэЈәИЎmgСщЖ·јУККБҝХфБуЛ®К№Ц®ИЬҪвЈ¬јУИлјёөО·УМӘЈ¬УГ0.1 mol/L өДСОЛбұкЧјИЬТәөО¶ЁЦБИЬТәУЙәмЙ«ұдОӘОЮЙ«Ј¬ПыәДСОЛбV1mLЈ»ФЩПтТСұдОЮЙ«өДИЬТәЦРјУИлјёөОјЧ»щіИЈ¬јМРшУГёГСОЛбөО¶ЁЦБИЬТәУЙ»ЖЙ«ұдіИЙ«Ј¬УЦПыәДСОЛбV2 mL.ЎЈ
ўЩКөСйКұУГөҪөДІЈБ§ТЗЖчУРЙХұӯЎўҪәН·өО№ЬЎўІЈБ§°фЎў____ЎЈ
ўЪЗуІРБф№ММеЦРMөДЦКБҝ·ЦКэ__________ЈЁУГә¬mЎўV1әН![]() өДҙъКэКҪұнКҫЈ©ЎЈ
өДҙъКэКҪұнКҫЈ©ЎЈ
ўЫИфУГјЧ»щіИЧчЦёКҫјБөО¶ЁҪбКшКұЈ¬өО¶Ё№ЬјвН·УРЖшЕЭЈ¬Ів¶ЁҪб№ыҪ«____МоЎ°Ж«ёЯ"ЎўЎ°Ж«өНЎұ»тЎ°І»ұдЎұЈ©ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com