
����Ŀ��������״���������г���84����Һ������������Ч�ɷ�ΪNaClO��Һ������Ҫ�ǻ��ڴ����ᣨHClO���������ԡ������ڵ�±��Ԫ���������������й㷺Ӧ�ã��ش��������⣺
��1����̬��ԭ�ӵĺ���۵����Ų�ʽΪ__________��HClO����������ԭ�ӵ��ӻ��������Ϊ__________��
��2������ũҩ��ԭ��PSCl3�У�P��S��Cl�ĵ縺���ɴ�С��˳��Ϊ_________��
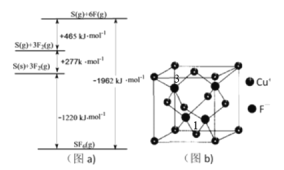
��3������Cl���ڵ�Ԫ��S��F���仯����SF6���㷺������ѹ�����豸�ľ�Ե���ʡ�SF6��һ�ֹ��ۻ������ͨ��������Born��Haberѭ��������������ͼ��a��������ؼ��ܣ���S��F���ļ���Ϊ__________��
��������γɵ���״������ṹ��ͼ��C�����仯ѧʽΪ__________��
��4��CuCl���۵�Ϊ426�棬�ۻ�ʱ���������磻CuF���۵�Ϊ908�档
��CuF���۵��CuCl�ĸߣ�ԭ����_________��
�ڹ�ҵ�Ͻ�CuCl����KCN��Һ�����Ƴɶ�ͭҺ����ͭҺ������ﻯѧʽΪ__________��д��һ�������廥Ϊ�ȵ�����������ӵĵ���ʽ__________��
��CuF������ͼ��b���������߳�Ϊa nm����Cu����F������ľ���Ϊ________����Mg��mol��1��ʾCuF��Ħ��������NA��ʾ�����ӵ�������ֵ����CuF������ܶ�Ϊ______g��cm��3��
���Ծ�������Ϊ��λ���Ƚ���������ϵ���Ա�ʾ�����и�ԭ�ӵ�λ�ã�����ԭ�ӷ������꣬����ͼ��b��������1������Ϊ��![]() ��
��![]() ��0����������3������Ϊ__________��
��0����������3������Ϊ__________��
���𰸡�3s23p5 sp3 Cl��S��P 327kJmol-1 (SO3)n CuCl�Ƿ��Ӿ��壬CuF�����Ӿ��� K3Cu(CN)4 ![]()
![]() nm
nm ![]()
![]()
��������
��1����̬��ԭ�����������Ӳ㣬����������Ϊ7���������۵����Ų�ʽΪ3s23p5��HClO���ӵ�����ԭ��Ϊ��ԭ�ӣ������ӻ�������ۣ����ӻ��������Ϊ sp3��
��2��ͬ����Ԫ�ش����ң��縺��Խ��Խǿ����P��S��Cl�ĵ縺���ɴ�С��˳��ΪCl��S��P��
��3������ͼ��a����֪6F(g)+S(g)=SF6(g) H=-1962 kJ/mol����S��F���ļ���Ϊ1962 kJ/mol��6��327kJmol-1��
����ͼ��C����֪��������γɵ���״���������ɶ��SO3���ӹ��ɵĽṹ�����仯ѧʽΪ(SO3)n��
��4������ΪCuF�����Ӿ��壬CuCl�Ƿ��Ӿ��壬��CuF���۵��CuCl�ĸߡ�
�ڽ�CuCl����KCN��Һ���γ�����Cu+�ṩ�չ�������������ӣ���λ��Ϊ4�����仯ѧʽΪK3Cu(CN)4�������廥Ϊ�ȵ�����������ӿ���ΪNO+������ʽΪ![]() ��
��
����ͼ��b����ʾ�������߳�Ϊa nm�������Խ��߳���Ϊ![]() nm��������Cu����F������ľ���Ϊ��Խ��ߵ�
nm��������Cu����F������ľ���Ϊ��Խ��ߵ�![]() ������Ϊ
������Ϊ![]() nm����������4��Cu����F���������ܶȹ�ʽ����֪
nm����������4��Cu����F���������ܶȹ�ʽ����֪![]() ��
��
�ܽ��þ����г�8�ȷݣ���8��С�����壬����3λ�����Ϸ�����������ģ���������1������Ϊ��![]() ��
��![]() ��0����������3������Ϊ
��0����������3������Ϊ![]() ��
��


 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͭ����������ʹ�õĽ���֮һ���䵥�ʼ���������й㷺����;��
��1����̬ͭԭ�Ӻ�����________�������෴�ĵ��ӡ�
��2����ͭ��ͭ������Ǧ��Ԫ�ذ�һ�������������ɵĺϽ𡣵�һ������I1(Sn)____________I1(Pb)(����ڡ���С�ڡ�)��
��3�����Ƶ�Cu(OH)2�ܹ��ܽ���Ũ��ˮ�У���Ӧ�����ӷ���ʽ��____________________________________��
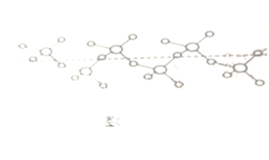
��4������ͭƬ�������Ӧ���ҹ��о���Ա����Ȳ����Ϊԭ�ϣ����������״�ͨ����ѧ�������ȫ̼���ϡ�ʯīȲ��Ĥ(�ṹƬ����ͼ��ʾ)���������˹���ѧ�ϳ�̼ͬ���������������ʯīȲ��̼ԭ��_________________________���ӻ���ʽ��

��5��CuCl��������Һ������CO�γ��Ȼ��ʻ���ͭ��I���������ڶ����ⶨ����������CO�ĺ������Ȼ��ʻ���ͭ��I���к�___________������Ŀ��
��6��Cu2O�����ڰ뵼����ϡ�
��Cu2O����(��ͼ��ʾ)�У�Oԭ�ӵ���λ��Ϊ________________��aλ��Cu+����Ϊ(0.25��0.25��0.75)����bλ��Cu+����_______________________��
��Cu2S��Cu2O�������ƾ���ṹ�������ߵ��۵���Cu2O��Cu2S��_________(��ߡ��͡�)�������ԭ��___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��FΪԭ�������������������Ԫ�أ�����A��B��C��D��EΪ������Ԫ�أ�FΪ��������Ԫ�أ�F����ǰ�������е縺����С��Ԫ�ء���֪��Aԭ�ӵĺ������������Ӳ�����ȣ�BԪ��ԭ�ӵĺ���p��������s��������1����Cԭ�ӵĵ�һ�����ĵ�����ΪI1��738 kJ��mol-1��I2��1 451 kJ��mol-1��I3��7 733 kJ��mol-1��I4��10 540 kJ��mol-1��Dԭ�Ӻ�������p���Ϊȫ������������EԪ�ص������������������IJ�Ϊ4��
��1��д��EԪ�������ڱ���λ�ã�________��DԪ�ص�ԭ�ӵĺ�������Ų�ʽ��__________________��
��2��ijͬѧ������Ŀ��Ϣ�����յ�֪ʶ����C�ĺ�������Ų�Ϊ![]() ��ͬѧ�����Ĺ��ʽΥ����________��
��ͬѧ�����Ĺ��ʽΥ����________��
��3����֪BA5Ϊ���ӻ����д�������ʽ��________��
��4��DE3����ԭ���ӻ���ʽΪ________����ռ乹��Ϊ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ֲ��ӷ��͵ijɷ�֮һ�����Ľṹ��ʽΪ![]() �����������в���ȷ���ǣ� ��
�����������в���ȷ���ǣ� ��
A. ���л������ʽΪC9H10O
B. 1mol������������1mol��ˮ������Ӧ
C. 1mol������������4mol����������Ӧ
D. �����ۿ���FeCl3 ��Һ����ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ԭ�����������������ǻ�����ѧ�о����ȵ���⡣
I.������ԭ����H2��ԭNO�����ķ�ӦΪ2NO��g����2H2��g��![]() N2��g����2H2O��g����
N2��g����2H2O��g����
��1����֪���ֻ�ѧ���ļ����������£�

2NO��g����2H2��g��![]() N2��g����2H2O��g�� ��H��__________kJ��mol��1
N2��g����2H2O��g�� ��H��__________kJ��mol��1
��2��2NO��g����2H2��g��![]() N2��g����2H2O��g���ķ�Ӧ���ʱ���ʽΪv��kc2��NO����c��H2����k�����ʳ�����ֻ���¶��йأ�����ѧ�о�����������Ӧ���������У�
N2��g����2H2O��g���ķ�Ӧ���ʱ���ʽΪv��kc2��NO����c��H2����k�����ʳ�����ֻ���¶��йأ�����ѧ�о�����������Ӧ���������У�
��Ӧ1����Ӧ������2NO��g����H2��g��![]() N2��g����H2O2��g����
N2��g����H2O2��g����
��Ӧ2����Ӧ�죩��H2O2��g����H2��g��![]() 2H2O��g��
2H2O��g��
����������Ӧ�У���ܽϴ���Ƿ�Ӧ__________���1����2������c��NO�����ܷ�Ӧ���ʵ�Ӱ��̶�__________c��H2��������ڡ���С�ڡ����ڡ�����
II.NH3��ԭ�����ں����ܱ������г���NH3��NO2����һ���¶��·�����Ӧ�� 8NH3��g����6NO2��g��![]() 7N2��g����12H2O��g��
7N2��g����12H2O��g��
��3�����б����÷�Ӧ�ﵽƽ��״̬����__________������ĸ����
A ��������ܶȱ��ֲ��� B NO2��N2����������֮��Ϊ6��7
C ���������c��N2����c��NO2�� D �������ѹǿ���ֲ���
III.CO��ԭ�������ø�Ч������������β���е�NO��CO��������Ӧ�� 2CO��g����2NO��g��![]() N2��g����2CO2��g�� ��H����2L�����ܱ������г���2 mol CO��2 mol NO�����NO��ת�������¶ȡ�ʱ��Ĺ�ϵ��ͼ��ʾ��
N2��g����2CO2��g�� ��H����2L�����ܱ������г���2 mol CO��2 mol NO�����NO��ת�������¶ȡ�ʱ��Ĺ�ϵ��ͼ��ʾ��
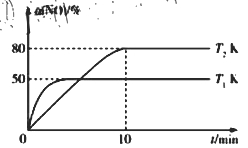
��4������˵����ȷ����_________������ĸ����
A ͼ���У�T1��T2 B ������Ӧ�ڸ��������Է�����
C 10minʱ��T2K�������淴Ӧ������� D ����NO��Ũ�ȣ���Ӧ���ת��������
��5��T2K�¶��£�0��10min����CO��ʾ��ƽ����Ӧ����v��CO����_________mol/L��1��min��1��T1K�¶��£�������Ӧ��ƽ�ⳣ��K��_________L��mol��1��
��6��T1K�¶��£���ƽ�����������ټ���2 mol N2��2 mol NO����ƽ��_________��������ƶ����������ƶ������ƶ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��1��3������ϩA�����ںϳ�CR��ҽҩ�м���K�����кϳ�K����·���£�

��֪��
I.�ȴ���D����Է���������113���ȵ���������ԼΪ62.8%���˴Ź����������֮��Ϊ2��1��
��.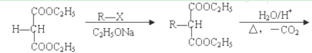 R-CH2COOH
R-CH2COOH
��1��B�������Ĺ�������__________��E��ϵͳ������������__________��
��2����Ӧ�ڵ�������__________������д���ٺ͢۵ķ�Ӧ����__________��__________��
��3��д��G��H�����з�Ӧ�Ļ�ѧ����ʽ________��
��4��K�Ľṹ��ʽΪ__________��
��5��д����G��2��̼ԭ�ӵ�ͬϵ�������ͬ���칹��Ľṹ��ʽ__________��
��6����AΪ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�CR��![]() ����·����
����·����
�����������̣����������ߺϳɹ���__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����25�桢101kPaʱ��C(s)��H2(g)��CH3COOH(l)��ȼ���ȷֱ�Ϊ393.5kJ/mol��285.8kJ/mol��870.3kJ/mol����2C(s)+2H2(g)+O2(g)= CH3COOH(l)���ʱ�Ϊ�� ��
A.-488.3kJ/molB.+488.3kJ/molC.+191kJ/molD.-191kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��b��![]() ����d��
����d��![]() ����p��
����p��![]() �ķ���ʽ��ΪC6H6������˵����ȷ����
�ķ���ʽ��ΪC6H6������˵����ȷ����
A. b��ͬ���칹��ֻ��d��p����B. b��d��p�Ķ��ȴ����ֻ������
C. b��d��p���������Ը��������Һ��ӦD. b��d��p��ֻ��b������ԭ�Ӵ���ͬһƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��ȷ����( )
A.���������Ũ���ᷴӦ��������MnO4-+ 8H+ + 4Cl-=Mn2++2Cl2��+4H2O
B.����̼������Һ��ͨ�����������̼��CO32-+ H2O+ CO2=2 HCO3-
C.ͭƬ��Ũ���Ṳ�ȣ�Cu + 4H++ SO42-![]() Cu2+ + SO2��+ 2H2O
Cu2+ + SO2��+ 2H2O
D.����ͭ��Һ�м��������ˮ��Cu2+ +4NH3H2O=Cu(NH3)42++4H2O
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com