
����Ŀ�����仯�����ڻ�ѧ��ҵ���������;����ش��������⣺
��1�����⻯��NaBH4�������Ҫ�����
��NaBH4��BԪ�صĻ��ϼ�Ϊ______��
![]() ��ҵ�Ͽ������������B(OCH3)3���⻯��NaH��Ӧ�Ʊ�NaBH4����Ӧ����һ�ֲ���Ϊ�״��ƣ�CH3ONa�����÷�Ӧ�Ļ�ѧ����ʽΪ______��
��ҵ�Ͽ������������B(OCH3)3���⻯��NaH��Ӧ�Ʊ�NaBH4����Ӧ����һ�ֲ���Ϊ�״��ƣ�CH3ONa�����÷�Ӧ�Ļ�ѧ����ʽΪ______��
��NaBH4��ˮ��Ӧ����NaBO2��H2���÷�Ӧ���ɵ����������뻹ԭ��������ʵ���֮��Ϊ______��
��2����ҵ���������![]() ��Ҫ�ɷ�ΪMg2B2O5��H2O��Fe3O4����������
��Ҫ�ɷ�ΪMg2B2O5��H2O��Fe3O4����������![]() ��FeO��CaO��
��FeO��CaO��![]() ��
��![]() ��
��![]() Ϊԭ���Ʊ�����B�Ĺ���������ͼ��ʾ��
Ϊԭ���Ʊ�����B�Ĺ���������ͼ��ʾ��

��֪��
�������� | Fe3+ | Al3+ |
��ʼ������pH | 2.7 | 3.1 |
������ȫ��pH | 3.7 | 4.9 |
![]() ��������ʱ���������ʯ�����Ŀ��Ϊ______��
��������ʱ���������ʯ�����Ŀ��Ϊ______��
![]() ����1����Ҫ�ɷ�Ϊ______��
����1����Ҫ�ɷ�Ϊ______��
![]() ���������ӡ�ʱ���ȼ�H2O2��Һ����Ŀ��Ϊ______��Ȼ���ٵ�����Һ��pH��5.0��Ŀ����______��
���������ӡ�ʱ���ȼ�H2O2��Һ����Ŀ��Ϊ______��Ȼ���ٵ�����Һ��pH��5.0��Ŀ����______��
![]() �ƵõĴ�����һ��������������BI3��BI3���ȷֽ���Եõ������ĵ������ֽ�
�ƵõĴ�����һ��������������BI3��BI3���ȷֽ���Եõ������ĵ������ֽ�![]() �����Ƴɵ�BI3��ȫ�ֽ⣬���ɵ�
�����Ƴɵ�BI3��ȫ�ֽ⣬���ɵ�![]() ��0.30mol��L��1Na2S2O3��Һ�ζ�
��0.30mol��L��1Na2S2O3��Һ�ζ� ���յ㣬����18.00mL Na2S2O3��Һ��ʢװNa2S2O3��ҺӦ��______
���յ㣬����18.00mL Na2S2O3��Һ��ʢװNa2S2O3��ҺӦ��______![]() ���ʽ����ʽ��
���ʽ����ʽ��![]() �ζ��ܣ��ô�����Ʒ�Ĵ���Ϊ______��
�ζ��ܣ��ô�����Ʒ�Ĵ���Ϊ______��
���𰸡�+3 4NaH+B(OCH3)3��NaBH4+3CH3ONa 1:1 ����Ӵ�������ӿ췴Ӧ���� SiO2��CaSO4 �����е�![]() ����Ϊ
����Ϊ![]() ʹFe3��ת��ΪFe(OH)3������Al3��ת��ΪAl(OH)3��������ȥ ��ʽ 97.2%
ʹFe3��ת��ΪFe(OH)3������Al3��ת��ΪAl(OH)3��������ȥ ��ʽ 97.2%
��������
��1����NaBH4��Na��+1�ۣ�H�ǣ�1�ۣ���BԪ�صĻ��ϼۣ����ݻ��ϼ۴�����Ϊ0���ó�BΪ+3�ۡ�
![]() ��ҵ�Ͽ������������B(OCH3)3���⻯��NaH��Ӧ�Ʊ�NaBH4����Ӧ����һ�ֲ���Ϊ�״��ƣ�CH3ONa�����÷�Ӧ�Ļ�ѧ����ʽΪ4NaH+B(OCH3)3��NaBH4+3CH3ONa ��
��ҵ�Ͽ������������B(OCH3)3���⻯��NaH��Ӧ�Ʊ�NaBH4����Ӧ����һ�ֲ���Ϊ�״��ƣ�CH3ONa�����÷�Ӧ�Ļ�ѧ����ʽΪ4NaH+B(OCH3)3��NaBH4+3CH3ONa ��
��NaBH4��ˮ��Ӧ����NaBO2��H2��NaBH4��2H2O��NaBO2��4H2����NaBH4��H���������õ��������ˮ���ⱻ��ԭ���õ���ԭ����÷�Ӧ���ɵ����������뻹ԭ��������ʵ���֮��Ϊ1:1��
��2�����������Ҫ�ɷ�ΪMg2B2O5��H2O��Fe3O4����������Fe2O3��FeO��CaO��Al2O3��SiO2�ȣ�Ϊԭ���Ʊ����ᣨH3BO3���������̿�֪���������ܽ�ֻ��SiO2���ܣ�Mg2B2O5��H2O+2H2SO4��2H3BO3+2MgSO4��CaOת��Ϊ����ˮ��CaSO4�����������ӡ����ȼ�H2O2��Һ������������ת��Ϊ�����ӣ�������Һ��pHԼΪ5��ʹ�����ӡ������Ӿ�ת��Ϊ������������Ϊ��������������������Ȼ������Ũ������ȴ�ᾧ�����˷����H3BO3��
�١�������ʱ���������ʯ�����Ŀ��Ϊ����Ӵ�������ӿ췴Ӧ���ʡ�
�ڼ������ܽ�ֻ��SiO2���ܣ�Mg2B2O5��H2O+2H2SO4��2H3BO3+2MgSO4��CaOת��Ϊ����ˮ��CaSO4������Fe3O4�Ĵ��ԣ��ɽ���ӡ��������з��롣���������л�ʣ���������SiO2��CaSO4������1����Ҫ�ɷ�ΪSiO2��CaSO4��
�ۡ��������ӡ����ȼ�H2O2��Һ����Ŀ��Ϊ�����е�![]() ����Ϊ
����Ϊ![]() ��Ȼ���ٵ�����Һ��pH��5.0��Ŀ����ʹFe3��ת��ΪFe(OH)3������Al3��ת��ΪAl(OH)3��������ȥ��
��Ȼ���ٵ�����Һ��pH��5.0��Ŀ����ʹFe3��ת��ΪFe(OH)3������Al3��ת��ΪAl(OH)3��������ȥ��
��Na2S2O3��Һ�ʼ��ԣ�Ӧ���ڼ�ʽ�ζ����У���������Ƶ����ʵ���Ϊ��0.30mol��L��1��0.018L=0.0054mol�����ݹ�ϵʽ��B��BI3��![]() I2��3S2O32������n��B��=
I2��3S2O32������n��B��=![]() n��S2O32����=0.0018mol���������Ϊ��10.81g��mol��1��0.0018mol=0.01944g����������ĺ���Ϊ��
n��S2O32����=0.0018mol���������Ϊ��10.81g��mol��1��0.0018mol=0.01944g����������ĺ���Ϊ��![]() ��100%=97.2%��
��100%=97.2%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I2��KI��Һ�д�������ƽ�⣺I2(aq) + I��(aq) ![]() I3��(aq)��ijI2��KI�����Һ�У�I3�������ʵ���Ũ��c(I3��)���¶�T�Ĺ�ϵ��ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������˵����ȷ����
I3��(aq)��ijI2��KI�����Һ�У�I3�������ʵ���Ũ��c(I3��)���¶�T�Ĺ�ϵ��ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������˵����ȷ����
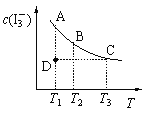
A.��Ӧ I2(aq) + I��(aq) ![]() I3��(aq)����H��0
I3��(aq)����H��0
B.״̬A��״̬B��ȣ�״̬A��c(I2)��
C.���¶�ΪT1��T2����Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2����K1��K2
D.����Ӧ���е�״̬Dʱ��һ����v����v��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾװ�ã��������ܱ������ڷֱ���������H2��O2�Ļ�������ڿ��ƶ��Ļ������ߣ��ڱ�״��������H2��O2�Ļ�������ȼ���������������ָ�ԭ�¶Ⱥ����һ�ͣ�������������룬��ԭ��H2��O2���������ӽ��� �� ��

��2��7 ��5��4 ��4��5 ��7��2
A.�٢�B.�ڢ�C.�ۢ�D.�٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������һ����������ѪС��ۼ���ҩ�����ԭ�ϵIJ�ͬ����ҩ��ĺϳ�·��ͨ����������������2���ȱ���ȩΪԭ�ϵĺϳ�·�����£�

��1��D��E����X���ʵĽṹ��ʽ��____________��
��2��C��D��Ӧ����D�IJ���ƫ�ͣ������ԭ��______________________________________��3��д��C�ۺϳɸ߷��ӻ�����ķ���ʽ___________________________________��
��4��A��ת����F����C7H7NO2��д��ͬʱ������������F������ͬ���칹��Ľṹ��ʽ��__________��
�ٺ����ױ��������к���-COO-�ṹ��
��1H-NMR����ʾ�����к��б������ұ����������ֲ�ͬ��ѧ��������ԭ�ӡ�
��5����֪��![]()
д������ϩ���״�Ϊ�л�ԭ���Ʊ�������![]() �ĺϳ�·������ͼ_____________________�����Լ���ѡ�����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ_____________________�����Լ���ѡ�����ϳ�·������ͼʾ�����£�![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�����л������������Ϊ��Ǧ���͵Ŀ����������C����������Ϊ68.2%����H����������Ϊ13.6%��
��1��������л�����������ʽ��Ҫ��д��������̣���___________
��2��________����������������������ȷ�����л�������ķ���ʽ������ȷ�������л���ķ���ʽΪ________________��������______________
��3��������ͺ˴Ź���������ʾ�÷�������4��������д����ṹ��ʽ___________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.1mol/L��CH3COOH��Һ�д������µ���ƽ�⣺CH3COOH![]() CH3COO-+H+�����ڸ�ƽ�⣬����������ȷ���� ( )
CH3COO-+H+�����ڸ�ƽ�⣬����������ȷ���� ( )
A.��������NaOH���壬ƽ��������Ӧ�����ƶ�
B.��ˮ����Ӧ��������ƽ�����淴Ӧ�����ƶ�
C.�μ�����0.1mol/L HCl��Һ����Һ��c(H+)����
D.��������CH3COONa���壬ƽ��������Ӧ�����ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ����1������ͭ������ܽ������ܽ���̽�����ʵ���ҳ�����ˮ�����Լӿ��ܽ����ʣ���������������ǣ��������ּ�Ҫ˵��ԭ��_______________��
��2��ϡNa2S��Һ��һ�ָ�����ζ������AlCl3��Һ������ζ�Ӿ磬�����ӷ���ʽ��ʾ��ζ�Ӿ�����������Ļ�ѧ��Ӧ______________��
��������������ԭ��Ӧ��MnO4-+5Fe2++8H+ = Mn2++5Fe3++4H2O�������õζ��ķ����ⶨFeSO4������������ʵ�鲽�����£��ٳ����̷���Ʒ�����100 mL������Һ����ȡһ���������Һ������ƿ�У�������һ���������ᣬ�۽���Ũ�ȵ�KMnO4��Һװ��ζ����У�����Һ����a mL�����ܵζ�����Һ���ζ��յ�ʱ���ζ��ܵ�Һ�����b mL�����ظ��ζ�2��3�Ρ�
��1�����֪���ζ������յ㣿__________�����в����ᵼ�²ⶨ���ƫ�͵���_______��
A��ʢ����Һ�ĵζ���������ˮϴ�Ӻ�δ�ñ�Һ��ϴ��װҺ�ζ�
B����ƿ������ˮϴ�Ӻ�δ�ô���Һ��ϴ
C����ȡ��Һ����ʱ���ζ�ǰƽ�ӣ��ζ����յ����
D���ζ�ǰ�ζ��ܼ��촦������δ�ų����ζ���������ʧ
��2������ÿ���������������ޣ�������ʵ���У�����ʹ�õ���____________��
A��������ƽ B����Ͳ C����ʽ�ζ��� D����ʽ�ζ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶��£�Ũ�ȶ���1 mol��L��1����������X2��Y2�����ܱ������з�Ӧ��������Z����Ӧ2 min��òμӷ�Ӧ��X2Ϊ0.6 mol��L��1����Y2�ı仯��ʾ�ķ�Ӧ����v(Y2)��0.1 mol��L��1�� min��1�����ɵ�c(Z)Ϊ0.4 mol��L��1����÷�Ӧ�Ļ�ѧ����ʽ��( )
A.X2��2Y2![]() 2XY2B.3X2��Y2
2XY2B.3X2��Y2![]() 2X3Y
2X3Y
C.X2��3Y2![]() 2XY3D.2X2��Y2
2XY3D.2X2��Y2![]() 2X2Y
2X2Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڰ�ˮ�д��ڵ���ƽ�⣺NH3��H2O ![]() NH4+ + OH��������������������ƽ�������ƶ�����( )��
NH4+ + OH��������������������ƽ�������ƶ�����( )��
�ټ�NH4Cl���� �ڼ�NaOH��Һ ��ͨHCl �ܼ�CH3COOH��Һ �ݼ�ˮ
A.�٢ۢ�B.�٢ܢ�C.�٢ڢ�D.�ۢܢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com