
����Ŀ�������ݱ������ҹ����Ϻ��������������еĿ�ȼ���������ˮ����)�Բɻ�óɹ���������һ����Ҫ�Ļ���ԭ�ϡ�
��1����������������������ʵ���Ҫ��ʽ������������������¶��֣�
ˮ����������CH4(g)+H2O(g)![]() CO(g)+3H2(g) ��H1=+205.9kJ��mol-1 ��
CO(g)+3H2(g) ��H1=+205.9kJ��mol-1 ��
CO(g)+H2O(g)![]() CO2(g)+H2(g) ��H2=��41.2kJ��mol-1 ��
CO2(g)+H2(g) ��H2=��41.2kJ��mol-1 ��
������̼������CH4(g)+CO2(g)![]() 2CO(g)+2H2(g) ��H3 ��
2CO(g)+2H2(g) ��H3 ��
��Ӧ ���Է����е�������________________����H3 =_____________kJ��mol-1��
�������Ĺ̶�һֱ�ǿ�ѧ���о�����Ҫ���⣬�ϳɰ������˹��̵��Ƚϳ���ļ�������ԭ��ΪN2(g)+3H2(g)![]() 2NH3(g)
2NH3(g)
��2���ڲ�ͬ�¶ȡ�ѹǿ����ͬ���������£���ʼʱN2��H2�ֱ�Ϊ0.1mol��0.3molʱ��ƽ��������а��������������������ͼ��ʾ��
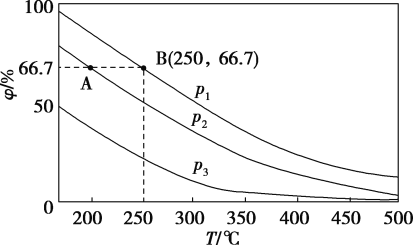
�����У�p1��p2��p3�ɴ�С��˳����________����ԭ����_________________��
�����ֱ���vA(N2)��vB(N2)��ʾ�ӷ�Ӧ��ʼ����ƽ��״̬A��Bʱ�Ļ�ѧ��Ӧ���ʣ���vA(N2)______ vB(N2)������>����<������=����
������250����p1Ϊ105Pa�����£���Ӧ�ﵽƽ��ʱ���������Ϊ1L�����������B��N2�ķ�ѹp(N2)Ϊ_________Pa (��ѹ=��ѹ�����ʵ�������������һλС��)��
���������������(S2O42��)Ϊý�飬ʹ�ü�ӵ绯ѧ��Ҳ�ɴ���ȼú�����е�NO��װ����ͼ��ʾ��
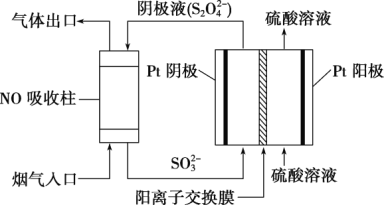
���������ĵ缫��ӦʽΪ_____________________________��
��NO����ת�������Ҫ����ΪNH4+����ͨ��ʱ��·��ת����0.3mole�������ͨ����������������յ�NO�ڱ�״���µ����Ϊ_______mL��
���𰸡� ���� +247.1 p1>p2>p3 �¶���ͬʱ������ѹǿ��ѧƽ��������Ӧ�����ƶ�����ƽ�������а����������Խ��ѹǿԽ�� < 8.3��103 2SO32-+4H++2e-=S2O42-+2H2O 1344
������������(1)��Ӧ ��CH4(g)+H2O(g)![]() CO(g)+3H2(g) ��H1=+205.9kJ��mol-1����H��0����S��0����ӦҪ�Է���������G=��H-T��S��0����Ҫ���£����Է����У����ݢ�CH4(g)+H2O(g)
CO(g)+3H2(g) ��H1=+205.9kJ��mol-1����H��0����S��0����ӦҪ�Է���������G=��H-T��S��0����Ҫ���£����Է����У����ݢ�CH4(g)+H2O(g)![]() CO(g)+3H2(g) ��H1=+205.9kJ��mol-1����CO(g)+H2O(g)
CO(g)+3H2(g) ��H1=+205.9kJ��mol-1����CO(g)+H2O(g)![]() CO2(g)+H2(g) ��H2=��41.2kJ��mol-1�����ݸ�˹���ɣ�����-�ڵã�CH4(g)+CO2(g)
CO2(g)+H2(g) ��H2=��41.2kJ��mol-1�����ݸ�˹���ɣ�����-�ڵã�CH4(g)+CO2(g)![]() 2CO(g)+2H2(g) ��H3=(+205.9kJ��mol-1)-(��41.2kJ��mol-1)=+247.1 kJ��mol-1���ʴ�Ϊ�������� +247.1��
2CO(g)+2H2(g) ��H3=(+205.9kJ��mol-1)-(��41.2kJ��mol-1)=+247.1 kJ��mol-1���ʴ�Ϊ�������� +247.1��
����(2)����N2+3H22NH3��֪������ѹǿ��ƽ�������ƶ�����ͼ���֪����ͬ�¶��£�ƽ��������а����������(��)ΪP1��P2��P3�����ѹǿ��ϵ��p1��p2��p3���ʴ�Ϊ��p1��p2��p3���¶���ͬʱ����ѹƽ�������ƶ�����ѹǿԽ��ƽ�������а����������Խ��ѹǿԽ��
���¶�Խ��ѹǿԽ��Ӧ����Խ��p1��p2����ͼ��֪��B��Ӧ���¶ȡ�ѹǿ����Ӧ���ʴʴ�Ϊ������
�� N2 + 3H2 2NH3
��ʼ(mol) 0.1 0.3 0
ת��(mol) x 3x 2x
ƽ��(mol) 0.1-x 0.3-3x 2x
![]() =0.667��x=0.08mol����B��N2�ķ�ѹp(N2)Ϊ
=0.667��x=0.08mol����B��N2�ķ�ѹp(N2)Ϊ![]() ��105Pa=8.3��103 Pa���ʴ�Ϊ��8.3��103��
��105Pa=8.3��103 Pa���ʴ�Ϊ��8.3��103��
��������ͼ��֪��������ͨ��Һ����Ҫ��SO32-��������Ҫ��S2O42-�������������缫��ӦʽΪ2SO32-+4H++2e-=S2O42-+2H2O���ʴ�Ϊ��2SO32-+4H++2e-=S2O42-+2H2O��
������NO����ת�������Ҫ����ΪNH4+��NO��NH4+��5e-������·��ת��ת��0.3mole-����Ҫ����NO0.06mol����״�������Ϊ0.06mol��22.4L/mol =1344mL���ʴ�Ϊ��1344��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[��ѧ����ѡ��5���л���ѧ����]��������(H)��һ�ֽ�Ѫ֬ҩ������Խ��ͼ����ܶȺ͵��ܶ�֬����ˮƽ�������߸��ܶ�֬���ף�ͨ�����Ƶ��̴��ķֲ����ɼ���CH��LDL��Ѫ�ܱڵij����������ܽ���ά������ֹѪС���������á���ͼ�Ǻϳɻ������ص�һ���·�����
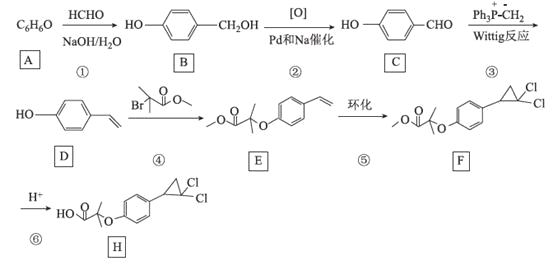
�ش��������⣺
(1)C�Ļ�ѧ����Ϊ______________________
(2)F�к��������ŵ�����Ϊ______________
(3)H�ķ���ʽΪ________________________
(4)��Ӧ�ٵķ�Ӧ����Ϊ___________����Ӧ�ܵĻ�ѧ����ʽΪ______________________
(5)MΪ![]() ��ͬ���칹�壬����NaHCO3��Һ��Ӧ�������壬��M�Ľṹ������____(�����������칹)������1HNMR����3��壬�ҷ����֮��Ϊ6��2��1�Ľṹ��ʽΪ_______
��ͬ���칹�壬����NaHCO3��Һ��Ӧ�������壬��M�Ľṹ������____(�����������칹)������1HNMR����3��壬�ҷ����֮��Ϊ6��2��1�Ľṹ��ʽΪ_______
(6)����Wittig��Ӧ������Ի�����Ϊԭ��(�����Լ���ѡ)���Ʊ�![]() �ĺϳ�·�ߣ�______________________��
�ĺϳ�·�ߣ�______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��
CH3CH2CH2+HCl![]() CH3CH2CH3 +Cl
CH3CH2CH3 +Cl![]() CH(CH3)2 + HCl
CH(CH3)2 + HCl
CH3CH2CH2+ HBr![]() CH3CH2CH3+Br
CH3CH2CH3+Br![]() CH(CH3)2+HBr
CH(CH3)2+HBr
����˵����ȷ���ǣ� ��
A.��H1=��H2+��H3-��H4
B.HCl��HBr�ļ��ܲ���Ա�ʾΪ��H1-��H3
C.��H2>��H4
D.����ʱ��������Cl��Ӧ���ʼӿ죬��Br��Ӧ���ʽ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ߴ���������Ϊ�ϳ���������Ԫ�������ϵ�ԭ�ϣ���ҵ�Ͽ�����Ȼ�������̷������̿���Fe��Al��Mg��Zn��Ni��Si��Ԫ�أ��Ʊ�����������ͼ��ʾ���ش��������⣺
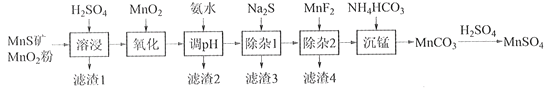
��ؽ�������[c0(Mn+)=0.1 mol��L1]�γ��������������pH��Χ���£�
�������� | Mn2+ | Fe2+ | Fe3+ | Al3+ | Mg2+ | Zn2+ | Ni2+ |
��ʼ������pH | 8.1 | 6.3 | 1.5 | 3.4 | 8.9 | 6.2 | 6.9 |
������ȫ��pH | 10.1 | 8.3 | 2.8 | 4.7 | 10.9 | 8.2 | 8.9 |
��1��������1������S��__________________________��д�����ܽ����ж������������̷�Ӧ�Ļ�ѧ����ʽ____________________________________________________��
��2����������������������MnO2�������ǽ�________________________��
��3������pH��������������Һ��pH��ΧӦ����Ϊ_______~6֮�䡣
��4��������1����Ŀ���dz�ȥZn2+��Ni2+��������3������Ҫ�ɷ���______________��
��5��������2����Ŀ��������MgF2������ȥMg2+������Һ��ȹ��ߣ�Mg2+��������ȫ��ԭ����_____________________________________________________________________��
��6��д���������������ӷ���ʽ___________________________________________________��
��7����״��������Ԫ���Ͽ���Ϊ����ӵ���������ϣ��仯ѧʽΪLiNixCoyMnz2������Ni��Co��Mn�Ļ��ϼ۷ֱ�Ϊ+2��+3��+4����x=y=![]() ʱ��z=___________��
ʱ��z=___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(VB2)��������ع���ʱ��ӦΪ��4VB2 ��11O2![]() 4B2O3 ��2V2O5 ���øõ��Ϊ��Դ��ѡ�ö��Ե缫���һ����������ͭ��Һ��ʵ��װ����ͼ��ʾ������·��ͨ��0.04mol����ʱ��Bװ���ڹ��ռ���0.448L���壨�������������˵������ȷ����
4B2O3 ��2V2O5 ���øõ��Ϊ��Դ��ѡ�ö��Ե缫���һ����������ͭ��Һ��ʵ��װ����ͼ��ʾ������·��ͨ��0.04mol����ʱ��Bװ���ڹ��ռ���0.448L���壨�������������˵������ȷ����

A. VB2�缫�����ĵ缫��ӦΪ�� 2VB2��11H2O �C 22e![]() V2O5��2B2O3��22H+
V2O5��2B2O3��22H+
B. ���·�е�����c�缫����VB2�缫
C. �������У�b�缫�������к�ɫ����������Ȼ�������ݲ���
D. ��Bװ���ڵ�Һ�����Ϊ200mL����CuSO4��Һ��Ũ��Ϊ0.05mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ��ش��������⣺
��1�������£�����Ũ�ȵ�Na2S2O3��Һ��������Һ��ϣ�2 min����Һ�����Գ��ֻ��ǣ���д����ط�Ӧ�����ӷ���ʽ��__________________________________________�������˻����Һ����50���ˮԡ�У�����ֻ��ǵ�ʱ�佫__________ (������������������������������)��
��2����֪![]() �ĵ���ƽ�ⳣ��
�ĵ���ƽ�ⳣ��![]() ����0.1mol/L
����0.1mol/L![]() ��Һ�еμ�NaOH��Һ��
��Һ�еμ�NaOH��Һ��![]() 1:18����ʱ��ҺPH=________��
1:18����ʱ��ҺPH=________��
��3��CO2��CH4�����������Ƶúϳ���CO��H2��
����֪������Ӧ����صĻ�ѧ�������������£�
��ѧ�� | C��H | C=O | H��H | C |
����/kJ��mol1 | 413 | 745 | 436 | 1075 |
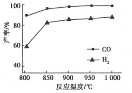
��÷�Ӧ���Ȼ�ѧ��Ӧ����ʽΪ��___________________________���ֱ���v L�����ܱ�����A�����ݣ���B����ѹ���ݻ��ɱ䣩�У�����CH4��CO2��1 mol�Ļ�����塣�������з�Ӧ��ƽ���ų������յ������϶����_______������A�� ����B ������
�ڰ�һ������ȼ���CH4��CO2���ں�ѹ�·�����Ӧ���¶ȶ�CO��H2���ʵ�Ӱ������ͼ��ʾ���˷�Ӧ��ѡ�¶�Ϊ900���ԭ����_________________________________��
��4����֪2CH3OH(g) ![]() CH3OCH3(g)��H2O(g)��ij�¶��µ�ƽ�ⳣ��K = 4�����¶��£����ܱ������м���CH3OH����Ӧ��20minʱ��ø���ֵ�Ũ�����£�
CH3OCH3(g)��H2O(g)��ij�¶��µ�ƽ�ⳣ��K = 4�����¶��£����ܱ������м���CH3OH����Ӧ��20minʱ��ø���ֵ�Ũ�����£�
���� | CH3OH | CH3OCH3 | H2O |
c / mol��L-1 | 0��4 | 0��1 | 0��1 |
�ٴ�ʱ����Ӧ��___________ (����������������)�ƶ������ܴﵽƽ�⡣
�ڴӼ���CH3OH��ʼ��Ӧ��20minʱ��CH3OCH3����������Ϊ_____________��
�۴ﵽƽ���CH3OH��Ũ��Ϊ_____________��
��5����ȡ���Ҷ����Ҷ����ķ�Ӧ����ʽ_______________________________
��6����һ����������֬����![]() �ṹ��ʽΪ
�ṹ��ʽΪ![]() ,д����ϳɵ������ϩ�裨CH2=CHCN��,2-������ϩ�⣬����Ҫ�ĵ��������ǣ�__________��
,д����ϳɵ������ϩ�裨CH2=CHCN��,2-������ϩ�⣬����Ҫ�ĵ��������ǣ�__________��
��7���л���R��C6H12O2����ϡ���Ṳ������A��B��A�ܹ�����������Ӧ��B���ܷ�����������Ӧ����ôR�Ľṹ��ʽΪ_________��
��8���ҹ�ѧ�߽��ʵ��������ģ�������о����ڽ����������ˮú���任[CO(g)+H2O(g)=CO2(g)+H2(g)]�ķ�Ӧ���̣���ͼ��ʾ�����������ڽ���������ϵ���������ע��
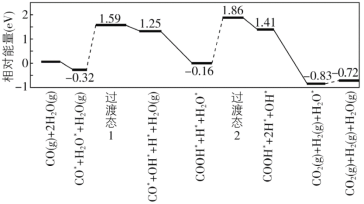
��֪ˮú���任����H________0������������������������С����������������������ݣ���ܣ�E��=_________eV��д���ò���Ļ�ѧ����ʽ_______________________��
��9��ij�л���A��C��H��O����Ԫ�����,��Է�������Ϊ90 .��9.0gA��ȫȼ�յIJ�������ͨ��������Ũ����ͼ�ʯ��,�ֱ�����5.4g��13.2g��A����NaHCO3��Һ��Ӧ,��2����A֮����ˮ�����ɰ�Ԫ��������.��A��һ�������·������۷�Ӧ�IJ���Ľṹ��ʽ��:_________________________
��10����֪��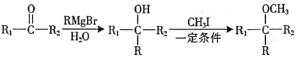 (R��ʾ������R1��R2��ʾ��������ԭ��)�������
(R��ʾ������R1��R2��ʾ��������ԭ��)�������![]() Ϊԭ���Ʊ�
Ϊԭ���Ʊ�![]() �ĺϳ�·��(�ṩCH3MgBr����Ҫ�����Լ�)_______________________________________________________________��
�ĺϳ�·��(�ṩCH3MgBr����Ҫ�����Լ�)_______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ���õ�ⷨ�����������Է�ˮ���õ�����![]() ��ԭ����ͼ��ʾ������˵������ȷ���ǣ� ��
��ԭ����ͼ��ʾ������˵������ȷ���ǣ� ��
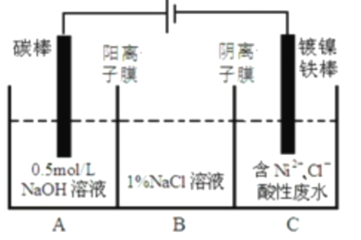
��֪����![]() ����������Һ�з���ˮ�⣻�������ԣ�
����������Һ�з���ˮ�⣻�������ԣ�![]() ����Ũ�ȣ�
����Ũ�ȣ�![]() ����Ũ�ȣ���
����Ũ�ȣ���
A.�������У�B��![]() ��Һ�����ʵ���Ũ�Ƚ���������
��Һ�����ʵ���Ũ�Ƚ���������
B.̼���Ϸ����ĵ缫��Ӧ��![]()
C.Ϊ�����![]() �IJ��ʣ�����������Ҫ���Ʒ�ˮ��
�IJ��ʣ�����������Ҫ���Ʒ�ˮ��![]()
D.��װ���е��ӵ�����Դ��������������������Դ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������(H3BO3)Ϊ���ᣬ��֪H3BO3������NaOH��Һ��Ӧ�����ӷ���ʽΪH3BO3+OH-=B(OH)4-��H3BO3����ͨ�����ķ����Ʊ����乤��ԭ����ͼ��ʾ(��Ĥ����Ĥ�ֱ�ֻ���������ӡ�������ͨ��)������˵��������ǣ� ��
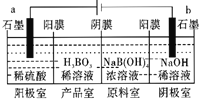
A.�����ĵ缫��ӦʽΪ��2H2O-4e-=O2��+4H+
B.��Ӧһ��ʱ���Ժ�������NaOH��ҺŨ������������H2SO4Ũ������
C.����·��ͨ��3mol����ʱ���ɵõ�1molH3BO3
D.�������У�B(OH)4-������Ĥ�����Ʒ�ϣ�Na+������Ĥ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��C6H14�ĸ���ͬ���칹������������������һ��ȡ�������Ŀ�ֱ��ǣ� ��
A.2������������4��һ�ȴ��� B.4������������1��һ�ȴ���
C.3������������5��һ�ȴ��� D.4������������4��һ�ȴ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com