
����Ŀ���ڸ����£� Al��Fe2O3�������ȷ�Ӧ��õ��Ĺ�����������Ҫ����Al2O3��Fe������������Fe2O3���Ӹ���Ʒ�й�����������Al2O3��������Fe��Fe2O3���������£�

��֪��NaAlO2 + CO2 + 2H2O = Al��OH��3�� + NaHCO3
�ش��������⣺
��1������ٵijɷ���__________����Һ�ڵ�������____________��
��2���������NaOH��Һʱ��������Ӧ�����ӷ���ʽ��__________��
��3����ɫ�������NaOH��Һ��Ӧ�����ӷ���ʽ��__________��
���𰸡�Fe��Fe2O3 NaHCO3 Al2O3 + 2OH-= 2AlO2- + H2O Al��OH��3 + OH-= AlO2- + 2H2O
��������
��������ӹ���NaOH��Һ�õ���Һ��ΪNaAlO2��NaOH�Ļ����Һ�������ΪFe��Fe2O3����Һ��ͨ������CO2�õ���Һ��ΪNaHCO3��Һ�������ΪAl��OH��3�������Դ˴��⡣
��������ӹ���NaOH��Һ�õ���Һ��ΪNaAlO2��NaOH�Ļ����Һ�������ΪFe��Fe2O3����Һ��ͨ������CO2�õ���Һ��ΪNaHCO3��Һ�������ΪAl��OH��3������Al��OH��3���ȷֽ�õ�Al2O3��
��1������ٵijɷ���Fe��Fe2O3����Һ�ڵ�����NaHCO3���ʴ�Ϊ��Fe��Fe2O3��NaHCO3��
��2���������NaOH��Һʱ��������Ӧ�����ӷ���ʽ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��3����ɫ�����ΪAl��OH��3��NaOH��Һ��Ӧ�����ӷ���ʽ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������[Cd(BrO3)2]�������������Ϻ�ӫ��ۡ���������(�ɷ�ΪCdO2��Fe2O3��FeO��������Al2O3��SiO2)Ϊԭ���Ʊ�[Cd(BrO3)2]����������:
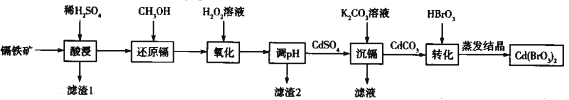
��֪��Cd(SO4)2����ˮ��
(1)Cd(BrO3)2��Cd�Ļ��ϼ�Ϊ__________
(2)���ʱ��Ϊ������ӵĽ�ȡ�ʿ��Բ�ȡ��ָʩ��__________(д�����ּ���)��
(3)��ԭ��ʱ,������ʹ����ʯ��ˮ����ǵ����壬�䷢����Ӧ�����ڷ���ʽΪ__________
(4)��H2O2��Һ����ʱ,�������뻹ԭ�������ʵ���֮��Ϊ__________
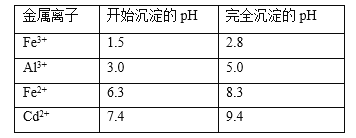
(5)��֪���ֽ������ӵ��������↑ʼ��������ȫ������pH���±�,��pHʱ��Ӧ������pH��Ϊ____,����2����Ҫ�ɷ�Ϊ_____(�ѧʽ)��
(6)ʵ�ʹ�ҵ������,��ʱ�����������ӽ�����֬�����ⶨ���Ӻ���Һ��Cd2+�ĺ���,��ԭ����: Cd2++2NaR=2Na++CdR2������NaRΪ�����ӽ�����֬�������£������Ӻ����Һ(��ʱ��ҺpH=6)���������ӽ�����֬�����Һ�е�Na+�Ƚ���ǰ������0.046g/L�����������Cd(OH)2��KspֵΪ____
(7)��֪��������CdO2�ĺ���Ϊ72%��������������Ԫ�ص������Ϊ8%����2t����������Ƶ�Cd(BrO3)2(��Է�������Ϊ368)_____Kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�ֵ��������Ҫ�����ڻ���ͭ(Cu2Te)������������(Cr2O3)�Լ������Ľ�(Au),����������ȡNa2Cr2O7��Һ������ͭ�ʹ��ڵ�,��ʵ���к����ϵ���Դ������,������������:
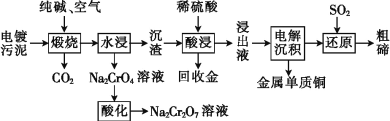
��֪:����ʱ,Cu2Te�����ķ�ӦΪCu2Te+2O2![]() 2CuO+TeO2��
2CuO+TeO2��
(1)����ʱ,Cr2O3������Ӧ�Ļ�ѧ����ʽΪ__________________��
(2)����Һ�г��˺���TeOSO4(�ڵ������в���Ӧ)��,�����ܺ���____(�ѧʽ)��
(3)��ҵ�����ظ�����(Na2Cr2O7)ĸҺ�����ظ����(K2Cr2O7)�Ĺ���������ͼ��ʾ:
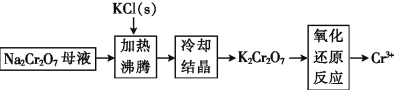
ͨ����ȴ�ᾧ����������K2Cr2O7��ԭ����__________________��
(4)�ⶨ��Ʒ��K2Cr2O7�����ķ�������:��ȡ��Ʒ����2.50 g���250 mL��Һ,����Һ��ȡ��25.00 mL����ƿ��,��������ϡ�����ữ��,�ټ��뼸��ָʾ��,��0.1000 mol��L-1���������(NH4)2Fe(SO4)2��Һ���еζ�,�ظ����ж���ʵ�顣(��֪Cr2![]() ����ԭΪCr3+)
����ԭΪCr3+)
��������ԭ�ζ������е����ӷ���ʽΪ________________��
��������ʵ������(NH4)2Fe(SO4)2��Һ��ƽ�����Ϊ25.00 mL,�����ò�Ʒ��K2Cr2O7�Ĵ���Ϊ_____%��[��֪M(K2Cr2O7)=294 g��mol-1,������������λ��Ч����]��
(5)����������K2Cr2O7����������ԭ��Ӧ��������Һ�г�����Cr3+��,������һ��Ũ�ȵ�Fe3+����,��ͨ���Ӽ��pH�ķ���ʹ����ת��Ϊ��������֪c(Cr3+)=3��10-5 mol��L-1,����Һ�п�ʼ����Cr(OH)3����ʱFe3+�Ƿ������ȫ?____(����������������)��{��֪:Ksp[Fe(OH)3]=4.0��10-38, Ksp[Cr(OH)3]=6.0��10-31}
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ִ��������ʻ��������г������������̼���������ɱ�����ܡ��衢�̡��ס����Ԫ�ء���ش������й����⣺
(1)��̬Mnԭ�ӵļ۵����Ų�ʽΪ___________��Mn2+��Fe2+�У���һ�����ܽϴ����__________���жϵ�������_____________________________________��
(2)̼Ԫ�س����γɳ�����������CO��CO2�⣬�����γ�C2O3(�ṹʽΪ![]() )��C2O3��̼ԭ�ӵ��ӻ��������Ϊ___________��CO2���ӵ����幹��Ϊ___________��
)��C2O3��̼ԭ�ӵ��ӻ��������Ϊ___________��CO2���ӵ����幹��Ϊ___________��
(3)̪ݼ�ܷ��ӵĽṹ��ʽ��ͼ��ʾ������������ԭ��ͨ����λ����ϵĵ�ԭ�ӵı����______(����1�� ��2�� ��3������4��)����C��H��OԪ�ص縺���ɴ�С��˳����_________________________
(4)̼���ε��ȷֽ������ھ����е������ӽ��̼����е������ӣ���̼����ֽ�ΪCO2���ӵĽ����MgCO3�ֽ��¶ȵ���CaCO3�������ԭ��_________________________��
(5)��������������NaCl�����ƣ�NaCl�ľ�����ͼ��ʾ�����ھ���ȱ�ݣ�ij�������������ʵ�����ΪFe0.9O�����а�����Fe2+��Fe3+�������߳�Ϊapm���þ�����ܶ�Ϊ��g��cm��3����a=___________(�г�����ʽ���ɣ���NA��ʾ�����ӵ�������ֵ)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������ԭ��Ӧ�ĵ���ת����Ŀ�ͷ���д��������
��1��2K2S + K2SO3 + 3H2SO4= 3K2SO4 + 3S��+ 3H2O _______������_______
��2��2KMnO4 ��5H2O2 + 3H2SO4 = K2SO4��2MnSO4��5O2�� �� 8H2O _______ ������_______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���п�Ժ������ѧ�����о�����һ�����³ɹ�ʵ���˼����Ч������ϩ����ͼ��ʾ�������ڴ����������⣬�ڲ�ͬ�¶��·ֱ��γ�![]() �����ɻ����������о����ɻ�:CH2ż����Ӧ������ϩ(�÷�Ӧ���̿���)
�����ɻ����������о����ɻ�:CH2ż����Ӧ������ϩ(�÷�Ӧ���̿���)

(1)��֪������ʵ�ȼ�������ϱ���ʾ��д�������Ʊ���ϩ���Ȼ�ѧ����ʽ______________��
(2)�ִ�ʯ�ͻ�������Ag����������ʵ����ϩ�������Ʊ�X(����ʽΪC2H4O������˫��)�÷�Ӧ�����������ԭ�Ӿ��ã���Ӧ������____________(��ṹ��ʽ)��
(3)��400��ʱ�����ʼ���Ϊ1L�ĺ�ѹ�ܱշ�Ӧ���г���1 molCH4������(1)�з�Ӧ�����ƽ����������C2H4���������Ϊ20.0������:
���ڸ��¶��£���ƽ�ⳣ��K��________��
������÷�Ӧ����ͨ�����ˮ����(���μӷ�Ӧ������400��)����C2H4�IJ��ʽ�_______(�������С�������䡱����ȷ����)��������__________________________________��
������Ӧ��������̶�����ͬѹǿ�¿ɵñ仯����ͼ��ʾ����ѹǿ�Ĺ�ϵ��____________��

��ʵ���Ʊ�C2H4ʱ��ͨ�����ڸ���Ӧ2CH4(g)![]() C2H6(g)+H2(g)����Ӧ����CH4��ʼ�����䣬��ͬ�¶���C2H6��C2H4������������¶ȵĹ�ϵ��������ͼ��ʾ��
C2H6(g)+H2(g)����Ӧ����CH4��ʼ�����䣬��ͬ�¶���C2H6��C2H4������������¶ȵĹ�ϵ��������ͼ��ʾ��

I.���¶ȸ���600��ʱ���п��ܵõ�һ�ֽ϶��˫̼�л��������������____________��
II.����400��ʱ��C2H4��C2H6����������ֱ�Ϊ20.0����6.0��������ΪCH4��H2������ϵ��CH4�����������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��ҡ�����ȡ300mLͬŨ�ȵ����ᣬ���벻ͬ������ͬһþ���Ͻ��ĩ��������ʵ�飬�й������б����£�
ʵ����� | �� | �� | �� |
�Ͻ�����/mg | 510 | 765 | 918 |
(��״��)�������/mL | 560 | 672 | 672 |
��1����������ʵ���Ũ���Ƕ���___��
��2���Ͻ���þ���������������Ƕ���___��___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���״���CH3OH������Ҫ���ܼ������ȼ�ϣ���ҵ����CO��H2��һ���������Ʊ�CH3OH�ķ�ӦΪCO(g)+2H2(g)![]() CH3OH(g)�� ��H��
CH3OH(g)�� ��H��
��1�������Ϊ1L�ĺ����ܱ������У�����2molCO��4molH2��һ�������·���������Ӧ�����CO(g)��CH3OH(g)��Ũ����ʱ��ı仯��ͼ����ʾ��
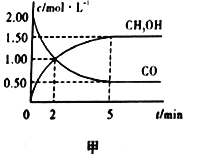
�ٴӷ�Ӧ��ʼ��5min����������ʾ��ƽ����Ӧ����v(H2)=________��
������˵����ȷ����________������ţ���
A. �ﵽƽ��ʱ��H2��ת����Ϊ75%
B. 5min��������ѹǿ���ٸı�
C. �ﵽƽ����ٳ����������Ӧ��������
D. 2minǰv(��)��v(��)��2min��v(��)��v(��)
��2��ij�¶��£���һ��ѹ�����зֱ����1.2molCO��1molH2���ﵽƽ��ʱ�������Ϊ2L���Һ���0.4molCH3OH(g)����÷�Ӧƽ�ⳣ����ֵΪ__________��
��3���״���һ�����͵���������ȼ�ϡ���֪H2(g)��CO(g)��CH3OH(l)��ȼ���ȷֱ�Ϊ285.8kJ/mol��283.0kJ/mol��726.5kJ/mol����״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪ________��
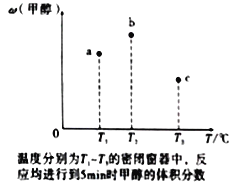
��4�������ݻ���Ϊ1L��a��b��c�����ܱ������������зֱ����1molCO��2molH2�Ļ�����壬�����¶ȣ����з�Ӧ�����������ݵĹ�ϵ��ͼ��ʾ��b�м״������������a�е�ԭ����____________���ﵽƽ��ʱ��a��b��c��CO��ת���ʴ�С��ϵΪ___________��
��5���״���Ϊһ��ȼ�ϻ�������ȼ�ϵ�ء����¶�Ϊ650���������ȼ�ϵ�����ü״���������CO2�Ļ����������Ӧ������缫����Li2CO3��Na2CO3�����������ʡ��õ�صĸ�����ӦʽΪ___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���0.100 0 mol![]() L��1��NaOH��Һ�ζ�20.00 mLδ֪Ũ�ȵ�ij��HX���ζ�������ͼ��ʾ������˵����ȷ����
L��1��NaOH��Һ�ζ�20.00 mLδ֪Ũ�ȵ�ij��HX���ζ�������ͼ��ʾ������˵����ȷ����

A. �ζ����̿��ü�����ָʾ��
B. �����£�HX�ĵ��볣��ԼΪ1��10��5
C. ��d��Һ�У�c(Na+)��c(X��)��c(OH��)��c(H+)
D. ��b��Һ�У�c(HX) + c(H+)��c(OH��) + c(X��)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com