
����Ŀ��������C��H��O���л���3.24 g��װ��Ԫ�ط���װ�ã�ͨ��������O2ʹ����ȫȼ�գ������ɵ���������ͨ���Ȼ��Ƹ����A�ͼ�ʯ�Ҹ����B�����A������������2.16g��B��������9.24g����֪���л������Է�������Ϊ108��
(1)ȼ�մ˻�����3.24g��������������������______________________��
(2)�û�����ķ���ʽΪ____________��
(3)�û�����1�����д���1�����������ں˴Ź�������ͼ����4�����շ塣��д�������ܵĽṹ��ʽ_______________��_______________��
���𰸡�8.16g C7H8O ![]()
![]()
��������
(1)A������������2.16gΪ����ˮ��������B��������9.24gΪ���ɶ�����̼�����������������غ��������������������
(2)�����л��ˮ��������̼�����ʵ���������ԭ���غ�����л��������C��Hԭ����Ŀ�������Է�������ȷ��Oԭ����Ŀ��
(3)�л��ﺬ�б������˴Ź������״����ĸ��壬˵������4��Hԭ�ӣ���Ϸ���ʽ��д���ܵĽṹ��
(1)�Թ�A��������2.16gΪ����ˮ��������B�ܼ�ʯ����CO2����9.24g���������غ㶨�ɣ���֪��������������m(O2)=2.16g+9.24g-3.24g=8.16g��
(2)�Թ�A��������2.16gΪ����ˮ����������ˮ�����ʵ���n(H2O)=2.16g��18g/mol=0.12mol����ʯ�Ҹ����B��������CO2������Ϊ9.24g��������̼�����ʵ���n(CO2)=9.24g��44g/mol=0.21mol�����л�������Ϊ3.24g����Է���������108�������л�������ʵ���Ϊn(�л���)=3.24g��108g/mol=0.03mol�������л�������У�N(C)=![]() =7�� N(H)=
=7�� N(H)=![]() =8�� N(O)=
=8�� N(O)=![]() =1����˸��л���ķ���ʽΪ��C7H8O��
=1����˸��л���ķ���ʽΪ��C7H8O��
(3)�����л��ﺬ�б������Һ˴Ź������״����ĸ��壬���ܵĽṹ��ʽΪ��![]() ��
��![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������л������HCl ��NaOH ��NH4Cl ��CH3COONa ��CH3COOH ��NH3H2O��Na2CO3���������ĸ�����ʾ�����ʻش��������⡣
(1)����Һ������Ũ�ȴ�С˳��Ϊ______________________�������ӷ���ʽ��ʾ����Һ�Լ��Ե�ԭ��_______________________________��
(2)�����£�pH=11�Ģܵ���Һ�У���ˮ���������c(OH-)=____________����֪�����¢ݺ͢ĵ��볣����Ϊ1.7��10-5 mol��L-1����Ӧ��CH3COOH+NH3H2O![]() CH3COO-+NH4++H2O��ƽ�ⳣ��Ϊ______________��
CH3COO-+NH4++H2O��ƽ�ⳣ��Ϊ______________��
(3)�����£�����pHֵ��ͬ�Ģٺ͢�������Һ������˵������ȷ����________��
A.������Һ��ˮ�ĵ���̶���ͬ B.c(CH3COO-)=c(Cl-)
C.c(CH3COOH)>c(HCl) D.���Ũ�ȵ�����������Һ��Ӧ���������ĵ������
(4)�����£���0.10 mol/L�Ģ���Һ��0.30 mol/L������Һ�������ϣ���ַ�Ӧ��ָ������£���Һ��pH=________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������л��ϳɡ���ص�����������Ҫ�����á�
I. ![]() ���Ʊ���Ӧ������ͼ��ʾ��
���Ʊ���Ӧ������ͼ��ʾ��

(1)�Ԫ����Ԫ�����ڱ��е�λ��_______________________��
(2)д��A�ĵ���ʽ___________________________��
(3)![]() ���л��ϳ��г��õĻ�ԭ������д����Ӧ�۵Ļ�ѧ����ʽ_________________��
���л��ϳ��г��õĻ�ԭ������д����Ӧ�۵Ļ�ѧ����ʽ_________________��
II.�������������������ӵ�ص���ѡ�缫���ϣ���������Ϊ������ʯīΪ����������������李��Ȼ�﮻����Һ��������������﮳�������800�����ҡ����������Χ�������Ƶá�������ӵ���У���Ҫһ���л��ۺ�����Ϊ������֮�������Ǩ�ƵĽ��ʣ����л��ۺ���ĵ���֮һ(��M��ʾ)�Ľṹ��ʽ���£�

��ش��������⣺
(4)�Ʊ���������ﮱ����ڶ��������Χ�н��У���ԭ����_______________��
(5)����������������﮵ĵ缫��ӦʽΪ___________________��
(6)д��M����������������Һ��Ӧ�Ļ�ѧ����ʽ_____________________��
(7)�õ�س��ʱ�����������������������������ŵ�ʱ�����ĵ缫��ӦʽΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һ��
��1�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ����������������Ϊ__mL���ζ��յ�ʱ�������ǣ����������һ������ʱ����ƿ����Һ��ɫ��___����30s���ָ�ԭɫ��

��2��ijѧ����������ʵ��ֱ��¼�й��������±���
�ζ����� | ������������ ��Һ�����/mL | 0.1000mol��L��1��������/mL | ||
�ζ�ǰ�̶� | �ζ���̶� | ��Һ��� | ||
��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
������ | 25.00 | 0.22 | 26.31 | 26.09 |
��ѡ�����к����������������������Һ���ʵ���Ũ��(����������4λ��Ч����)��c(NaOH)=__mol��L��1��
��3�����ڴ��������ʹ��������������������Һ��Ũ��ƫ�ߵ���__(����ĸ)��
A.�ζ�ǰ�ζ����������ݣ��ζ�����ʧ B.��ʽ�ζ�����ȡNaOH��Һʱ��δ������ϴ����
C.�ζ�ʱ�ﵽ�ζ��յ�ʱ���Ӷ��� D.��ƿȡ��NaOH����Һǰ������ˮϴ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʵ��������������ó��Ľ�����ȷ����
ѡ�� | ʵ����������� | �� �� |
A | ��һ��Ũ�ȵ�Na2SiO3 ��Һ��ͨ������CO2 ���壬 ���ְ�ɫ������ | H2SiO3 �����Ա�H2CO3������ǿ |
B | ������Fe(NO3)2��ˮ�ܽ�μ�ϡ�����ữ���ٵμ�KSCN��Һ����Һ���Ѫ��ɫ | Fe(NO3)2�ѱ��� |
C | �����£���ã�0.1mol��L-1 Na2SO3��Һ��pHԼΪ10��0.1mol��L-1 NaHSO3��Һ��pHԼΪ5�� | HSO3�� ���H+ ��������SO32����ǿ |
D | �ֱ���25mL��ˮ��25mL��ˮ�е���6��FeCl3 ������Һ��ǰ��Ϊ��ɫ������Ϊ���ɫ�� | �¶����ߣ�Fe3+��ˮ��̶����� |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�������Һ�и�����Ũ�ȹ�ϵ��ȷ����
A.pH��12�İ�ˮ��pH��2������������ϣ�c(Cl��)>c(NH4+)>c(H��)>c(OH��)
B.Ũ��Ϊ0.1mol��L��1��̼������Һ��c(Na��)��c(CO32��)��c(HCO3��)��c(H2CO3)
C.0.1mol��L��1��NaHS��Һ������Ũ�ȹ�ϵ��c(OH��)=c(H+)��c(S2��)+c(H2S)
D.������Һ��NaOH��Һ��Ϻ�������Һ�����ԣ�c(Na��)>c(CH3COO��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��PVAc��һ�־��������Ե���֬���ɺϳ���Ҫ�߷��Ӳ���M���ϳ�·�����£�
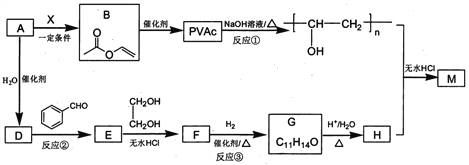
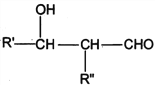
��֪��R��R�@��R�@�@ΪHԭ�ӻ�����
I. R'CHO+ R"CH2CHO![]()
![]()
![]()
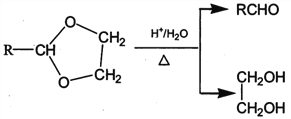
II. RCHO+![]()
![]()
��1����״���£�4.48L��̬��A��������5.2g����A�Ľṹ��ʽΪ___________________��
��2����֪A��BΪ�ӳɷ�Ӧ����X�Ľṹ��ʽΪ_______��B�й����ŵ�������_________��
��3����Ӧ�ٵĻ�ѧ����ʽΪ______________________��
��4��E��ʹ������Ȼ�̼��Һ��ɫ����Ӧ�ڵķ�Ӧ�Լ���������_______________________��
��5����Ӧ�۵Ļ�ѧ����ʽΪ____________________________��
��6����E��F��G��H��ת�������У��Ҷ�����������__________________________��
��7����֪M�������г������⣬��������Ԫ��״�ṹ����M�Ľṹ��ʽΪ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ؾ��壨K3[Fe(C2O4)3]��xH2O����һ�ֹ������ϣ���110�����ȫʧȥ�ᾧˮ��Ϊ�ⶨ�þ��������ĺ�����ijʵ��С����������ʵ�飺
�������IJⶨ
����һ������5.00 g�����������ؾ��壬���Ƴ�250 mL��Һ��
�������ȡ������Һ25.00 mL����ƿ�У���ϡH2SO4�ữ���μ�KMnO4��Һ�������ǡ��ȫ�������ɶ�����̼��ͬʱMnO4������ԭ��Mn2+����Ӧ�����Һ�м���һС��п�ۣ���������ɫ�պ���ʧ�����ˣ�ϴ�ӣ������˼�ϴ��������Һ�ռ�����ƿ�У���ʱ��Һ�Գ����ԡ�
����������0.010 mol/L KMnO4��Һ�ζ������������Һ���յ㣬����KMnO4��Һ20.02 mL���ζ���MnO4������ԭ��Mn2+���ظ���������������������ζ�����0.010 mol/L KMnO4��Һ�ֱ�Ϊ19.98 mL��20.00 mL����ش��������⣺
��1���ζ������У����������ҺӦʢװ��__________�ζ����У����ʽ����ʽ������
��2�������ӷ���ʽ��ʾ��������漰������ػ�ѧ��Ӧ��________________�� Zn + 2Fe3+ = 2Fe2+ + Zn2+��
��3���������еζ��յ���ж���____________________��
��4���ڲ�����У��������KMnO4��Һ�������������õ�������____________���ڲ������У����ζ�ǰ���Ӷ������ζ����Ӷ��������õ�������__________����ѡ�ƫ�͡�����ƫ�ߡ��������䡱��
��5��ʵ���øþ�����������������Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʯ����Ҫ�ɷ�BaCO3(��Ca2����Mg2����Fe3��������)��ʵ�������ö���ʯ�Ʊ�BaCl2��2H2O���������£�

��1������ʯ�������ȡǰ������ĥ��Ŀ����________��ʵ������37%����������15%�����ᣬ����Ͳ���ʹ�����������е�________��
a���ձ���b��500mL����ƿ��c����������d���ζ���
��2����֪��ͬ�������ӿ�ʼ�����ͳ�����ȫ��pH���£�

����NH3��H2O����pH=8�ɳ�ȥ_______ (�����ӷ���)����ʱ����Һ�и����ӵ�Ũ��Ϊ_______mol��L��1������NaOH��pH=12.5����Һ��ʣ�����������_______��ȫ������_____________ (�����ӷ���)���ֳ���������H2C2O4ʱӦ���������ԭ����___________������֪��Ksp(BaC2O4)=1.6��10��7,Ksp(CaC2O4)=2.3��10��9, Ksp��Fe(OH)3�� =2.6��10��39��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com