
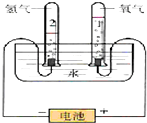
 ˮ�����౦�����Ȼ��Դ��Ҳ��һ����Ҫ�Ļ���ԭ�ϣ���ͼ�ǵ��ˮԭ����ʵ��װ��ͼ��
ˮ�����౦�����Ȼ��Դ��Ҳ��һ����Ҫ�Ļ���ԭ�ϣ���ͼ�ǵ��ˮԭ����ʵ��װ��ͼ�� ��
��| �� �� | ���ʵ��� | O2��H2������ȣ�ͬ��ͬѹ�£� | |
| O2 | 3.2g | 0.1mol | 1��2 |
| H2 | 0.4g | 0.2mol |
���� ��1��������Ӧ��2H2O$\frac{\underline{\;���\;}}{\;}$2H2��+O2����ת�Ƶ�����ĿΪ4e-��
��2������n=$\frac{m}{M}$����ˮ�����ʵ���������2H2O$\frac{\underline{\;���\;}}{\;}$2H2��+O2�����㣻
��3��������Ӧ��2Na+2 H2O�T2NaOH+H2������Ӧ����Һ������ΪNaOH�����ݷų���������NaOH��������������Һ����=ˮ������+�Ƶ�����-��������������������Һ��NaOH��������������n=$\frac{m}{M}$���Եõ�NaOH���ʵ�����Ҫ�������Һ�����ʵ����ʵ���Ũ�ȣ���Ҫ��Һ��������ڿ��Ի����Һ����������Ҫ��Һ�ܶȣ�
��4������n=$\frac{V}{{V}_{m}}$�����������ʵ��������ݷ���ʽ���������������ʵ������ٸ���m=nVm��������������
��� �⣺��1��������Ӧ��2H2O$\frac{\underline{\;���\;}}{\;}$2H2��+O2����ת�Ƶ�����ĿΪ4e-����˫���ű�ʾ�����ӵ�ת�Ʒ������ĿΪ�� ��
��
�ʴ�Ϊ�� ��
��
��2��3.6gH2O�����ʵ���Ϊ$\frac{3.6g}{18g/mol}$=0.2mol��
2H2O$\frac{\underline{\;���\;}}{\;}$2H2��+O2��
2 2 1
0.2mol 0.2mol 0.1mol
��������������Ϊ0.1mol��32g/mol=3.2g��ʧȥ��������Ϊ0.2mol��2g/mol=0.4g��
ͬ��ͬѹ��O2��H2�������Ϊ0.1mol��0.2mol=1��2��
�ʴ�Ϊ��
| �� �� | ���ʵ��� | O2��H2������ȣ�ͬ��ͬѹ�£� | |
| O2 | 3.2g | 0.1mol | 1��2 |
| H2 | 0.4g | 0.2mol |
���� ���⿼�黯ѧ����ʽ���㡢������ԭ��Ӧ����ҺŨ���йؼ���ȣ�ע�������õ����š�˫���ű�ʾ����ת����Ŀ�뷽��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ��ͨ���綫�������з��ظ���10��������ѧ���������棩 ���ͣ�ѡ����
����ָ����Ӧ�����ӷ���ʽ��ȷ����
A����ͭ����Ũ�����У�Cu+4H++2NO3-��Cu2++2NO2��+2H2O
B����FeBr2��Һ��ͨ�����������2Fe2++Cl2��2Fe3++2Cl-
C����Al2O3�м��백ˮ��Al2O3+2OH-��2 AlO2��+H2O
D��̼��Ũ������ȣ�C+2H++SO42����H2O+ SO2��+ CO2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����ϵ�һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
���ݱ�������˵����ȷ���ǣ���
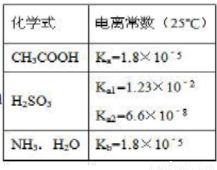
A��25��Cʱ��pH=3�Ĵ����pH=11�İ�ˮ�������Ϻ��Һ��ˮ�ĵ����DZ��ٽ���
B��Na2SO3��Һ�еμ��������ᣬ��Ӧ�����ӷ���ʽΪ��SO3 +2CH3COOH=SO2��+H2O+2CH3COO-
C�� NaHSO3��Һ�����ԣ�����ΪKw/Ka1>Ka2
D��0��1mol/L��CH3COOH��Һ���Ũ�ȵ������CH3COONa ��Ϻ���Һ�е����������¹�ϵ�� c (H+) +c (CH3C OOH) =c (CH3COO-
OOH) =c (CH3COO- ) +2c (OH-)
) +2c (OH-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH3��CuS��SO2��FeS | B�� | SO3��Cu2S��FeI2��FeCl3 | ||
| C�� | FeCl3��SO2��NO��Cu2S | D�� | FeCl2��FeS��SO2��FeI3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ɱ�-H2O | B�� | ˮ��-Ag | C�� | �ƾ�-C2H5OH | D�� | ����-NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ����I | ����II |
| A | п������Ա���ǿ | ���������װп��ɼ�����ʴ |
| B | Ba��OH��2�������ᷴӦ | Ba��OH��2����������θ����� |
| C | SiO2������������ | SiO2����ˮ��Ӧ���ɹ��� |
| D | H2O2�������� | H2O2��ʹ���Ը��������Һ��ɫ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | z=2 | B�� | 2s�������ڵ�ѹǿ�dz�ʼ��$\frac{7}{8}$�� | ||
| C�� | 2sʱC���������Ϊ$\frac{2}{7}$ | D�� | 2s��B��Ũ��Ϊ0.5mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �÷�Ӧ��Na2Sx����������NaClO�ǻ�ԭ�� | |
| B�� | Na2Sx�������Ӽ��ͼ��Թ��ۼ� | |
| C�� | 1molNa2Sx�μӷ�Ӧ����32mol����ת�� | |
| D�� | Na2Sx�е�X��ֵΪ2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 46 g��NO2��N2O4������庬�е�ԭ����Ϊ3NA | |
| B�� | 1 mol Na2O2������H2O��Ӧ��ת�Ƶĵ�����ΪNA | |
| C�� | ���ʵ���Ũ��Ϊ0.5 mol/L MgCl2��Һ������Cl-������ΪNA | |
| D�� | 2.7g�������������������������Һ��Ӧ������H2�ķ�������Ϊ0.15NA |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com