
����Ŀ���״�������������ϸ���У���ά�ֹ��������ݵı�Ҫ���ʣ�Ҳ����Ҫ�ķǽ���Ԫ�ء�
�ش��������⣺
(1)��̬��ԭ�ӵļ۵����Ų�ʽΪ________________��
(2)N��Pͬ����������,PF3��NH3������������ɽ����γ������,��NF3ȴ��������ɽ����γ������,��ԭ����____________________________________________��
(3)����Ϊ��Ԫ��,��ṹʽΪ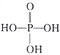 ��PO43-�Ŀռ乹��Ϊ____________��д��һ����PO43-��Ϊ�ȵ����������ڷǼ��Է��ӵĻ�ѧʽ��____________________��
��PO43-�Ŀռ乹��Ϊ____________��д��һ����PO43-��Ϊ�ȵ����������ڷǼ��Է��ӵĻ�ѧʽ��____________________��
(4)��������ȿɷ������Ӽ���ˮ���ɽ�����(H4P2O7)���������Լ��߾����ᣬ�����������ǿ�������ԭ����______________________________________________________��
(5)����(BP)���ܵ��߶ȹ�ע����ĥͿ�ϡ�BP��B��Pԭ�Ӿ��γɹ��ۼ������д�����λ��,��λ�����ṩ�µ��ӶԵ���____________(��Ԫ�ط���)ԭ��;����ľ���������____________������Bԭ�ӵ��ӻ���ʽ��____________�ӻ�,1molBP�к���____________molB-P����
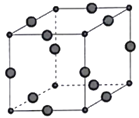
(6)Cu3P�ľ����ṹ��ͼ��ʾ,P3-����λ��Ϊ____________,Cu+�İ뾶Ϊapm,P3-�İ뾶Ϊbpm,�����ӵ�������ֵΪNA,��CuP������ܶ�Ϊ____________g��cm-3(�ú�a��b��NA�Ĵ���ʽ��ʾ)��
���𰸡� 3s23p3 Fԭ�ӵ縺��ǿ������Nԭ�ӵĵ���,ʹ�����Ը������Ӷ��γ���λ�� ���������� CCl4(��SiF4) �������з��ǻ�������Ŀ�������з��ǻ�������Ŀ�� P ԭ�Ӿ��� sp3 4 6 ![]() g/ cm3
g/ cm3
��������(1)��Ϊ15��Ԫ�أ���̬��ԭ�Ӻ�����15�����ӣ�����������Ϊ5���ʻ�̬��ԭ�ӵļ۵����Ų�ʽΪ3s23p3��(2)N��Pͬ����������,PF3��NH3������������ɽ����γ������,��NF3ȴ��������ɽ����γ������,��ԭ����Fԭ�ӵ縺��ǿ������Nԭ�ӵĵ���,ʹ�����Ը������Ӷ��γ���λ����(3) PO43-�Ŀռ乹��Ϊ���������Σ��ȵ�������ָ�۵�������ԭ�����������ԭ�Ӳ������ڣ���ͬ�ķ��ӡ����ӻ�ԭ���ţ���PO43-��Ϊ�ȵ����������ڷǼ��Է��ӵĻ�ѧʽ��CCl4��SiF4��(4)��������ȿɷ������Ӽ���ˮ���ɽ�����(H4P2O7)���������Լ��߾����ᣬ�����������ǿ�������ԭ���ǽ������з��ǻ�������Ŀ�������з��ǻ�������Ŀ�ࣻ(5) BP��B��Pԭ�Ӿ��γɹ��ۼ������д�����λ��,��λ�����ṩ�µ��ӶԵ���Pԭ��; ��3������BP������ĥͿ�ϣ������γɵľ���Ӧ����ԭ�Ӿ��塣Bԭ���γ�4�����ۼ�������ÿ����1molBP�����γ�4molB-P��������Bԭ�ӵ��ӻ���ʽ��sp3�ӻ���(6)����Cu3P�ľ����ṹ��֪��P3-�ھ����Ķ��㣬����λ��Ϊ6������ԭ�Ӿ�̯�����㣬ÿ����������![]() ��P3-��12
��P3-��12![]() ��Cu+�������߳�Ϊ2��a+b��pm,���Ϊ8��a+b��3pm3=8
��Cu+�������߳�Ϊ2��a+b��pm,���Ϊ8��a+b��3pm3=8![]() ��a+b��3cm3�����ܶ�Ϊ
��a+b��3cm3�����ܶ�Ϊ =
=![]() g/ cm3��
g/ cm3��


 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڿ��淴ӦmA(g)+nB(g)pC(g)+qD(g)+Q (Q>0)����m+n>p+q��ʹƽ��������Ӧ�����ƶ���������( )
A.���¡���ѹB.���¡���ѹC.���¡���ѹD.���¡���ѹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ͭ�ڳ������ܱ�ŨHNO3�ܽ⣬��ӦΪ:
Cu��4HNO3===Cu(NO3)2��2NO2��ʮ2H2O��
��1����˫���ŷ��������ת�Ƶķ������Ŀ________________________ ��
��2��������Ӧ�У���������______������������________����ԭ���뻹ԭ��������ʵ���֮��Ϊ________��
��3������1mol Cu����������ת�Ƶ��ӵ���ĿΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ���ڷ��ȷ�Ӧ���ǣ� ��
A.����ˮ�ķ�ӦB.̼��ƷֽⷴӦC.̿��ˮ������ӦD.ˮ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ��������п�����������������ϡ���ᷴӦ������2.8 L(���)������ԭ����������������(����)
A. 2 g B. 1 g C. 8 g D. 10 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������M����Ҫ���л��ϳ��м���,HΪ�߷��ӻ�����,��ϳ�·����ͼ��ʾ��

��֪���� (RΪ����);
(R����);
��2R-CH2CHO![]()
![]() ��
��
��ش��������⣺
(1)J������Ϊ____________��E�����������ŵ�����Ϊ____________��H�Ľṹ��ʽΪ____________��
(2)C��D�漰�ķ�Ӧ������____________________________��
(3)A��B+F�Ļ�ѧ����ʽΪ___________________________________��
(4)D��������Һ��Ӧ�Ļ�ѧ����ʽΪ______________________________________________��
(5)��������������M��ͬ���칹����____________��(�����������칹)��
�ٹ������������Ŀ��M��ͬ
�ڷ����к���1��-CH3��1��-CH2CH2-
�۲���-CH2CH2CH2-
(6)������ѧ֪ʶ��������Ϣ��д���Ա���ȩ��һ������Ϊԭ��(���Լ���ѡ)���Ʊ��л���ȩ(![]() )�ĺϳ�·�ߣ�_________________________________________________��
)�ĺϳ�·�ߣ�_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������ڵ���ʣ����ڸ��������²��ܵ������
A. Һ̬�Ȼ��� B. ���� C. �� D. ϡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)0.5 mol Na2CO3�к���___________��Na+��
(2)��������50 g�� HCl��NH3��CO2��O2�������壬����ͬ�¶Ⱥ���ͬѹǿ�����£����������____________��
(3)����90 mL 0.1 mol/L CuSO4��Һ����Ҫ����________g��
(4)������ԭ��Ӧ3S+6KOH=2K2S+K2SO3+3H2O�У��������뻹ԭ�������ʵ���֮��Ϊ_____________������Ӧ��������0.6molS����Ӧ��ת�Ƶĵ���Ϊ________mol��
(5)��������10�����ʣ���H2O����Mg����CH3COOH����NaOH����CuSO4��5H2O����ơ���C2H5OH�������ᣬ(�����������Ӧ�Ŀո���)���У�����ǿ����ʵ���_____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com