
����Ŀ������![]() ��ʽ̼���
��ʽ̼���![]() ������ˮ
������ˮ![]() ��ѧʽΪ
��ѧʽΪ![]() �������Ʊ���������
�������Ʊ���������![]() ��ԭ�ϡ�
��ԭ�ϡ�![]() �۷��������������¾��л�ԭ�ԡ�һ���Ʊ�����
�۷��������������¾��л�ԭ�ԡ�һ���Ʊ�����![]() ��ʽ̼��立������£�
��ʽ̼��立������£�
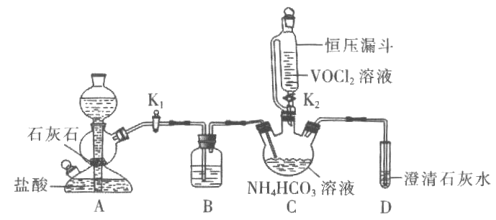
��ش��������⣺
(1)![]() װ��ʢװ���Լ���________
װ��ʢװ���Լ���________![]() ������
������![]() ��A�з�Ӧ�����ӷ���ʽΪ________����Է�Һ©������ѹ©���ŵ���________��
��A�з�Ӧ�����ӷ���ʽΪ________����Է�Һ©������ѹ©���ŵ���________��
(2)ʵ��ʱ���ȹر�![]() ����
����![]() ����________
����________![]() ��ʵ������
��ʵ������![]() ʱ����________
ʱ����________![]() ��ʵ�����
��ʵ�����![]() ��
��
(3)ʵ����Ϻ�Cװ���з����Ʒ�IJ���������________![]() ���������
���������![]() ��
��
(4)�ⶨ�ֲ�Ʒ�з��ĺ�����ʵ�鲽�����£�
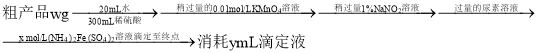
��֪���ζ���ӦΪ![]()
![]() �ôֲ�Ʒ�з������������ı���ʽΪ________
�ôֲ�Ʒ�з������������ı���ʽΪ________![]() ��
��
![]() ���ζ�ǰ���Ӷ������յ�ʱ���Ӷ��������ý��________����ƫ��������ƫ����������Ӱ����
���ζ�ǰ���Ӷ������յ�ʱ���Ӷ��������ý��________����ƫ��������ƫ����������Ӱ����![]() ��
��
���𰸡�����![]() ��Һ
��Һ ![]() ����Һ��˳������
����Һ��˳������ ![]() ����Һ����� �ر�
����Һ����� �ر�![]() ����
����![]() ����
���� ![]() ƫ��
ƫ��
��������
![]() װ����ʵ������ȡ������̼��װ�ã�������̼�л��е�����������Ҫ��HCl����ʵ��IJ�ƷΪ��ʽ̼���Σ�����HCl��Ӧ������Ҫ�ñ���
װ����ʵ������ȡ������̼��װ�ã�������̼�л��е�����������Ҫ��HCl����ʵ��IJ�ƷΪ��ʽ̼���Σ�����HCl��Ӧ������Ҫ�ñ���![]() ��Һ��ȥ
��Һ��ȥ![]() �����л��е�����HCl����ѹ©���ŵ���ƽ������ѹǿ��ʹ©����Һ����˳�����£�
�����л��е�����HCl����ѹ©���ŵ���ƽ������ѹǿ��ʹ©����Һ����˳�����£�
![]() ����ɿ�֪
����ɿ�֪![]() �۷��������������¾��л�ԭ�ԣ��ױ�����������C��ͨ��
�۷��������������¾��л�ԭ�ԣ��ױ�����������C��ͨ��![]() ��Ŀ���dz�ȥ��������ֹ
��Ŀ���dz�ȥ��������ֹ![]() ������Ʒ��Dװ�õij���ʯ��ˮ�������ڼ���
������Ʒ��Dװ�õij���ʯ��ˮ�������ڼ���![]() ����D����Һ�����ʱ������C�п������ž�����ʱ�ر�
����D����Һ�����ʱ������C�п������ž�����ʱ�ر�![]() ����
����![]() ����
����![]() ��Һ���뵽
��Һ���뵽![]() ��Һ�з�����Ӧ��
��Һ�з�����Ӧ��
![]() ����ɿ�֪����
����ɿ�֪����![]() ��ʽ̼���������ˮ����ʵ����Ϻ�Cװ���з����Ʒ�IJ��������ǹ��ˣ�
��ʽ̼���������ˮ����ʵ����Ϻ�Cװ���з����Ʒ�IJ��������ǹ��ˣ�
(4) �ټ���![]() ��Һ������
��Һ������![]() ��ʽ̼�������Ϊ
��ʽ̼�������Ϊ![]() ����
����![]() ��ȥ������
��ȥ������![]() �������س�ȥ������
�������س�ȥ������![]() ��������
��������![]() ��Һ��
��Һ��![]() ������Ӧ��
������Ӧ��![]() ���ɵζ���Ӧʽ֪��
���ɵζ���Ӧʽ֪��![]() mol����ôֲ�Ʒ�з���������������ʼ���Ӷ�����
mol����ôֲ�Ʒ�з���������������ʼ���Ӷ�����![]() ƫ���յ㸩�Ӷ�����
ƫ���յ㸩�Ӷ�����![]() ƫС����
ƫС����![]() ƫС����ý��ƫ�͡�
ƫС����ý��ƫ�͡�
![]() װ����ʵ������ȡ������̼��װ�ã�A�з�Ӧ�����ӷ���ʽΪ��
װ����ʵ������ȡ������̼��װ�ã�A�з�Ӧ�����ӷ���ʽΪ��![]() ��������̼�л��е�����������Ҫ��HCl����ʵ��IJ�ƷΪ��ʽ̼���Σ�����HCl��Ӧ������Ҫ�ñ���
��������̼�л��е�����������Ҫ��HCl����ʵ��IJ�ƷΪ��ʽ̼���Σ�����HCl��Ӧ������Ҫ�ñ���![]() ��Һ��ȥ
��Һ��ȥ![]() �����л��е�����HCl����Է�Һ©������ѹ©���ŵ���ƽ������ѹǿ��ʹ©����Һ����˳�����£�
�����л��е�����HCl����Է�Һ©������ѹ©���ŵ���ƽ������ѹǿ��ʹ©����Һ����˳�����£�
![]() ����ɿ�֪
����ɿ�֪![]() �۷��������������¾��л�ԭ�ԣ��ױ�����������C��ͨ��
�۷��������������¾��л�ԭ�ԣ��ױ�����������C��ͨ��![]() ��Ŀ���dz�ȥ��������ֹ
��Ŀ���dz�ȥ��������ֹ![]() ������Ʒ��Dװ�õij���ʯ��ˮ�������ڼ���
������Ʒ��Dװ�õij���ʯ��ˮ�������ڼ���![]() ����D����Һ�����ʱ������C�п������ž�����ʱ�ر�
����D����Һ�����ʱ������C�п������ž�����ʱ�ر�![]() ����
����![]() ����
����![]() ��Һ���뵽
��Һ���뵽![]() ��Һ�з�����Ӧ��
��Һ�з�����Ӧ��
![]() ����ɿ�֪����
����ɿ�֪����![]() ��ʽ̼���������ˮ����ʵ����Ϻ�Cװ���з����Ʒ�IJ��������ǹ��ˣ�
��ʽ̼���������ˮ����ʵ����Ϻ�Cװ���з����Ʒ�IJ��������ǹ��ˣ�
![]() ����
����![]() ��Һ������
��Һ������![]() ��ʽ̼�������Ϊ
��ʽ̼�������Ϊ![]() ����
����![]() ��ȥ������
��ȥ������![]() �������س�ȥ������
�������س�ȥ������![]() ��������
��������![]() ��Һ��
��Һ��![]() ������Ӧ��
������Ӧ��![]() ���ɵζ���Ӧʽ֪��
���ɵζ���Ӧʽ֪��![]() mol����ôֲ�Ʒ�з�����������
mol����ôֲ�Ʒ�з�����������![]() ��
��
![]() ��ʼ���Ӷ�����
��ʼ���Ӷ�����![]() ƫ���յ㸩�Ӷ�����
ƫ���յ㸩�Ӷ�����![]() ƫС����
ƫС����![]() ƫС����ý��ƫ�͡�
ƫС����ý��ƫ�͡�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ú������Һ�����ִ���Դ��ҵ���ص㿼�ǵ���Դ�ۺ����÷������������������Ϊ��ú����ˮú��������ǰ�Ƚ����е�Һ������Ϊ��ú����CH3OH��
��1����֪��CO2(g)��3H2(g)===CH3OH(g)��H2O(g) ��H1
2CO(g)��O2(g)===2CO2(g) ��H2
2H2(g)��O2(g)===2H2O(g) ��H3
��ӦCO(g)��2H2(g)===CH3OH(g)����H��______��
��2����ͼ�Ǹ÷�Ӧ�ڲ�ͬ�¶���CO��ת������ʱ��仯�����ߡ�

��T1��T2�¶��µ�ƽ�ⳣ����С��ϵ��K1________K2(������������������������)��
����CO�ϳɼ״�ʱ��CO��250 ����300 ����350 ���´ﵽƽ��ʱת������ѹǿ�Ĺ�ϵ��������ͼ��ʾ��������c����ʾ���¶�Ϊ________ �档ʵ����������������250 ����1.3��104 kPa���ң�ѡ���ѹǿ��������____________��

�������йظ÷�Ӧ��˵����ȷ����________(�����)��
A�����¡����������£��������ڵ�ѹǿ���ٷ����仯������淴Ӧ�ﵽƽ��
B��һ�������£�H2������������CO���������ʵ�2��ʱ�����淴Ӧ�ﵽƽ��
C��ʹ�ú��ʵĴ��������̴ﵽƽ���ʱ�䲢���CH3OH�IJ���
D��ij�¶��£���2 mol CO��6 mol H2����2 L�ܱ������У���ַ�Ӧ���ﵽƽ����c(CO)��0.2 mol��L��1����CO��ת����Ϊ80%
��3��һ���¶��£���2 L�̶�������ܱ������м���1 mol CH3OH(g)��������Ӧ��CH3OH(g) ![]() CO(g)��2H2(g)��H2�����ʵ�����ʱ��仯��������ͼ��ʾ��
CO(g)��2H2(g)��H2�����ʵ�����ʱ��仯��������ͼ��ʾ��

0��2 min�ڵ�ƽ����Ӧ����v(CH3OH)��__________�����¶��£���ӦCO(g)��2H2(g) ![]() CH3OH(g)��ƽ�ⳣ��K��__________����ͬ�¶��£���һ�����������м���һ������CO(g)��H2������CO(g)��2H2(g)
CH3OH(g)��ƽ�ⳣ��K��__________����ͬ�¶��£���һ�����������м���һ������CO(g)��H2������CO(g)��2H2(g) ![]() CH3OH(g)�ķ�Ӧ��ijʱ�̲����ϵ�и����ʵ���Ũ�����£�C��CO��=0.25 mol��L��1��C��H2��=1.0 mol��L��1��C��CH3OH��=0.75 mol��L��1�����ʱ�÷�Ӧ_____���У�����������Ӧ�����������淴Ӧ��������������ƽ��״̬������
CH3OH(g)�ķ�Ӧ��ijʱ�̲����ϵ�и����ʵ���Ũ�����£�C��CO��=0.25 mol��L��1��C��H2��=1.0 mol��L��1��C��CH3OH��=0.75 mol��L��1�����ʱ�÷�Ӧ_____���У�����������Ӧ�����������淴Ӧ��������������ƽ��״̬������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ǿ����ǿ����ϡ��Һ�ﷴӦ���к��ȿɱ�ʾΪ��H��(aq)��OH��(aq)=H2O(l) ��H����57.3kJ��mol-1�����з�Ӧ��
CH3COOH(aq)��NaOH(aq)=CH3COONa(aq)��H2O(l) ��H����Q1kJ��mol-1
![]() H2SO4(Ũ)��NaOH(aq)=
H2SO4(Ũ)��NaOH(aq)=![]() Na2SO4(aq)��H2O(l) ��H����Q2kJ��mol-1
Na2SO4(aq)��H2O(l) ��H����Q2kJ��mol-1
HNO3(aq)��NaOH(aq)=NaNO3(aq)��H2O(l) ��H����Q3kJ��mol-1
������Ӧ������Һ�н��У�������Q1��Q2��Q3�Ĺ�ϵ��ȷ���ǣ� ��
A.Q2>Q3>Q1B.Q2>Q1>Q3C.Q1��Q2��Q3D.Q2��Q3>Q1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
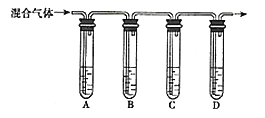
����Ŀ����ͼ��ʵ�����Ʊ�����������ʵ��װ�ã��Իش��������⣺
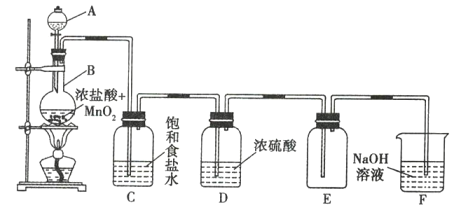
��1��д���������������ƣ�A __________________ ��B ________________ ��
��2��д���Ʊ������ķ�Ӧ�����ӷ���ʽ�� __________________ ��
��3��װ��C�������� __________________ ��װ��D�������� __________________ ��
��4��װ��F�������� __________________ ���û�ѧ����ʽ��ʾ����
��5��ʵ�����ʱ����Ҫ�ȳ�ȥB��C֮��ĵ��ܣ�Ȼ���ٳ����ƾ��ơ����ȳ����ƾ��ƣ�������ȥB��C֮��ĵ��ܣ���ᵼ�� __________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͬ����ѧС����ʵ�����ж�![]() ����̽�����ش��������⣺
����̽�����ش��������⣺
(1)![]() ѧ��������ͼEװ���Ʊ�
ѧ��������ͼEװ���Ʊ�![]() ������Ӧ�ķ���������������е�������________
������Ӧ�ķ���������������е�������________

A.������ ![]() ��ԭ��
��ԭ�� ![]() ����
���� ![]() ����
����
![]() ѧ������
ѧ������![]() ��δ��
��δ��![]() Ϊԭ����ȡ
Ϊԭ����ȡ![]() ����Ӧ�Ļ�ѧ����ʽ�ǣ�___
����Ӧ�Ļ�ѧ����ʽ�ǣ�___
![]() �����ռ�һƿ�����
�����ռ�һƿ�����![]() ��ѡ����ͼ�е�A��B��C��Dװ�ã�������˳��
��ѡ����ͼ�е�A��B��C��Dװ�ã�������˳��![]() ������������Сд��ĸ��ʾ�����ظ�ѡ��
������������Сд��ĸ��ʾ�����ظ�ѡ��![]() Ϊ��_________
��_________
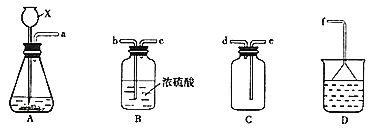
(2)ѧ������Ũ![]() �����Ƿ�Ӧ���õ��������к���
�����Ƿ�Ӧ���õ��������к���![]() ��
��![]() ����ѡ����ͼ�е�װ�ã���֤��������д���
����ѡ����ͼ�е�װ�ã���֤��������д���![]() ��
��![]() �����У���֤������
�����У���֤������![]() ��������_____��
��������_____��
(3)ѧ����ʵ���У����Թ��ڲ����˴�����ɫ���塣��������֪��
![]() ��ɫ������ܺ���CuO��CuS��
��ɫ������ܺ���CuO��CuS��![]() ��
��
![]() ��
��![]() ������ϡ���ᡢϡ���ᣬ�������¿�����ϡ���ᡣ
������ϡ���ᡢϡ���ᣬ�������¿�����ϡ���ᡣ
![]() ����
���� ![]() ����Һ�еμ�
����Һ�еμ�![]() ��Һ���ܲ������ɫ������
��Һ���ܲ������ɫ������
��ȡ������ɫ����������ϡ���ᣬ������Ժ��ٵμ�![]() ��Һ��δ�����ɫ�������ɴ����ý�����_____________________����ȡ������ɫ��������������ϡ���Ტ���ȣ����ֺ�ɫ����������ȫ�ܽ⣬��Һ��Ϊ��ɫ������ɫ�������ɣ��ܿڴ����ֺ���ɫ����д��CuS��������ϡ��������ӷ���ʽ��__________Ϊ�ⶨ��ɫ������
��Һ��δ�����ɫ�������ɴ����ý�����_____________________����ȡ������ɫ��������������ϡ���Ტ���ȣ����ֺ�ɫ����������ȫ�ܽ⣬��Һ��Ϊ��ɫ������ɫ�������ɣ��ܿڴ����ֺ���ɫ����д��CuS��������ϡ��������ӷ���ʽ��__________Ϊ�ⶨ��ɫ������![]() �İٷֺ�����ȡ
�İٷֺ�����ȡ![]() ��ɫ��������������Һ����
��ɫ��������������Һ���� ![]() ��Һ������������Ӧ���£�
��Һ������������Ӧ���£�![]()
![]() ,��Ӧ�������Һ���Ͼ�
,��Ӧ�������Һ���Ͼ�![]() �������ĸ��������Һǡ����
�������ĸ��������Һǡ����![]() ��Һ��ȫ��Ӧ����������
��Һ��ȫ��Ӧ����������![]() ����������Ϊ ___________
����������Ϊ ___________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
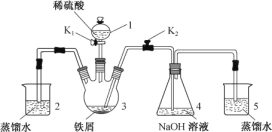
����Ŀ��ijͬѧ�����ͼװ��(�������Ѽ��)�Ʊ�Fe(OH)2��ɫ������
��ش�
(1) ����1������________��װ��5������________��
(2) ʵ�鿪ʼʱ���ر�K2����K1����Ӧһ��ʱ����ٴ�K2���ر�K1������3����Һ���ܽ���4�С���Ϊװ����һ���Ľ���ʹ��Һ�ܽ���4��________��
(3) װ�øĽ���3�з�Ӧ����Һѹ��4�У���4�������˻���ɫ��������ʵ��������̷���û�в�����ɫ������ԭ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������£���һ������ɱ���ܱ������г���2 mol������A��1 mol������B������Ӧ��2A(g)��B(g)![]() 3C(g)����H>0��t1ʱ�̷�Ӧ�ﵽƽ�⣬�����C�������е��������Ϊ��1��t2ʱ�̸ı�ijһ����(������������)��C�������е���������ı仯��ͼ��ʾ����t2ʱ�̸ı��������(����)
3C(g)����H>0��t1ʱ�̷�Ӧ�ﵽƽ�⣬�����C�������е��������Ϊ��1��t2ʱ�̸ı�ijһ����(������������)��C�������е���������ı仯��ͼ��ʾ����t2ʱ�̸ı��������(����)

A. ����1 mol����
B. ����1 mol B
C. ����ѹǿ
D. �����¶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ࡢ��֬�͵����ʶ����������Ӫ�����ʡ���ش��������⣺
(1)���Թ��м���![]() ���ۺ�
���ۺ�![]() 20%��
20%��![]() ��Һ������3~4min��Ȼ���ü�Һ�к��Թ��е�
��Һ������3~4min��Ȼ���ü�Һ�к��Թ��е�![]() ��Һ��
��Һ��
�ٵ�����ȫˮ�����ɵ��л���ķ���Ϊ___________________________��
����Ҫ��������Ѿ�������ˮ�⣬��ȡ����������Һ�������м���___________________________�����Լ������ƣ������Ⱥ��ٸ���ʵ�������жϣ���Ҫ�������û����ȫˮ�⣬��ȡ����������Һ�������м��뼸�ε�ˮ���ɹ۲쵽___________________________��
(2)��֬��������ͨ��ˮ������___________________________�ͱ��������������ֽ⣬Ϊ�����ṩ������
(3)Ϊ�˼���ij��ɫ��֯Ʒ�ijɷ��Dz�˿���ǡ�����˿����ͨ��ѡ�õķ�����_____________������ţ���
A.�μ�ϡ���� B.�μ�Ũ���� C.�μӾƾ� D.�ڻ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ľ����Ҫ�ɷ���̼��ء��ִӲ�ľ������ȡ���Σ�����ʵ��������е�S![]() ��C
��C![]() ��Cl-��
��Cl-��
(1)�Ӳ�ľ������ȡ���ε�ʵ�����˳�����£��ٳ�����Ʒ�����ܽ�ͳ�������______����_______������ȴ�ᾧ��
(2)��������ƽ(ָ�����ϵ�)������Ʒʱ����ָ��ƫ���ұߣ����ʾ___��
A.�����أ���Ʒ�� B.�����ᣬ������
C.�����أ������� D.�����ᣬ��Ʒ��
(3)�ڽ��Тڢۢܲ���ʱ��Ҫ�õ��������������÷ֱ���_____��______��______��
(4)���Ƶõ�������������Թܣ�������ˮ�ܽⲢ����Һ�ֳ����ݣ���װ����֧�Թ��
���ڵ�һ֧�Թ������ϡ���ᣬ�ɹ۲���___���ɣ�֤����Һ����_____��
���ڵڶ�֧�Թ����������ϡ������ټ����Ȼ�����Һ���ɹ۲���________���ɣ�֤����Һ����__________��
���ڵ���֧�Թ����������ϡ������ټ�����������Һ���ɹ۲���______���ɣ�֤����Һ����Cl-��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com