
����Ŀ����֪:�����¼װ���CH3NH2���ĵ��볣��kb��pkb=-lgkb=3.4��CH3NH2+H2O![]() CH3NH3++OH-������˵������ȷ���ǣ� ��
CH3NH3++OH-������˵������ȷ���ǣ� ��
A. (CH3NH3)2SO4��Һ������Ũ��:c(CH3NH3+)>c(SO42-)>c(H+)>c(OH-)
B. �����£�pH=3��������Һ��pH=11��CH3NH2��Һ�������ϣ������Һ������
C. �ñ�Ũ�ȵ�����ζ�δ֪Ũ�ȵ�CH3NH2��Һ��ʵ���У�ѡ�������ָʾ��
D. ��������CH3NH2��Һ�μ�ϡ������c(CH3NH2)=c(CH3NH3+)ʱ����ҺpH=10.6
���𰸡�B
��������
�װ�(CH3NH2)�ĵ��볣��Kb��pKb=-lg Kb=3.4��CH3NH2+H2O![]() CH3NH3++OH-����˼װ������백����A.���� (CH3NH3)2SO4��Һ�����������Һ�����жϣ�B. CH3NH2��������ݳ����£�pH=3��������Һ��pH=11��CH3NH2��Һ�������ϣ��װ�ʣ������жϣ�C. �ñ�Ũ�ȵ�����ζ�δ֪Ũ�ȵ�CH3NH2��Һ��ʵ���У�ǡ����ȫ�к�ʱ��Һ�������ԣ��ݴ˷����жϣ�D. ����Kb��
CH3NH3++OH-����˼װ������백����A.���� (CH3NH3)2SO4��Һ�����������Һ�����жϣ�B. CH3NH2��������ݳ����£�pH=3��������Һ��pH=11��CH3NH2��Һ�������ϣ��װ�ʣ������жϣ�C. �ñ�Ũ�ȵ�����ζ�δ֪Ũ�ȵ�CH3NH2��Һ��ʵ���У�ǡ����ȫ�к�ʱ��Һ�������ԣ��ݴ˷����жϣ�D. ����Kb��![]() ��c(OH-)�����c(CH3NH2)=c(CH3NH3+)�������
��c(OH-)�����c(CH3NH2)=c(CH3NH3+)�������
A. �װ�(CH3NH2)�ĵ��볣��Kb��pKb=-lg Kb=3.4��CH3NH2+H2O![]() CH3NH3++OH-�� ���(CH3NH3)2SO4��Һ�����������Һ��CH3NH3+��H2O
CH3NH3++OH-�� ���(CH3NH3)2SO4��Һ�����������Һ��CH3NH3+��H2O![]() CH3NH2H2O��H+������Ũ��:c(CH3NH3+)>c(SO42-)>c(H+)>c(OH-)����A��ȷ��B. CH3NH2����������£�pH=3��������Һ��pH=11��CH3NH2��Һ���������д����ļװ�����ʣ�࣬�����Һ�ʼ��ԣ���B����C. �ñ�Ũ�ȵ�����ζ�δ֪Ũ�ȵ�CH3NH2��Һ��ʵ���У�ǡ����ȫ�к�ʱ��Һ�������ԣ�Ӧ��ѡ�������ָʾ������C��ȷ��D. Kb��
CH3NH2H2O��H+������Ũ��:c(CH3NH3+)>c(SO42-)>c(H+)>c(OH-)����A��ȷ��B. CH3NH2����������£�pH=3��������Һ��pH=11��CH3NH2��Һ���������д����ļװ�����ʣ�࣬�����Һ�ʼ��ԣ���B����C. �ñ�Ũ�ȵ�����ζ�δ֪Ũ�ȵ�CH3NH2��Һ��ʵ���У�ǡ����ȫ�к�ʱ��Һ�������ԣ�Ӧ��ѡ�������ָʾ������C��ȷ��D. Kb��![]() ��c(OH-)��pH��14��pOH��14��3.4��10.6����D��ȷ����ѡB��
��c(OH-)��pH��14��pOH��14��3.4��10.6����D��ȷ����ѡB��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и�����������Һ���ܴ���������ǣ� ��
A.Cu2+��K+��SO42-��NO3-B.Na+��H+��Cl-��HCO3-
C.Na+��NH4+��OH-��Cl-D.H+��K+��SO42-��CO32-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijУ�Ļ�ѧ��ȤС����������ʵ��װ��̽��������������ʣ�E��װ�г�������,��Eװ�������IJ������ܿɸ�����Ҫ�任���̡�
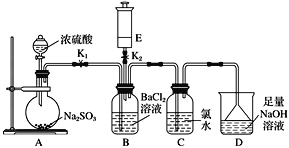
ʵ�鿪ʼʱ�ر�K2��K1���ӷ�Һ©��������ƿ�ڼ���Ũ���ᡣ
(1)A�з�����Ӧ�Ļ�ѧ����ʽ�ǣ�________________________________________��B�е�������_____________________________________��
(2)C�е�������______________д��C�з�Ӧ�Ļ�ѧ����ʽ��___________________________________��
(3)D��������________________________����Ӧ�����ӷ���ʽΪ__________________________________��
(4)A�з�Ӧ��ɺر�K1����K2����E�е���ɫ���建��ע��B�У��а�ɫ��������:
����E����ɫ�д̼�����ζ���壬���ķ���ʽΪ________�������İ�ɫ������_______________(�ѧʽ)
����E����ɫ��ζ���壬���ķ���ʽΪ________�� �����İ�ɫ������_______________(�ѧʽ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͼΪ���ڱ���һ���֣���֪A��B��C��D��E����Ԫ��ԭ�Ӻ����85�����ӣ�Eԭ�Ӻ������ĸ����Ӳ㣬BԪ����( )
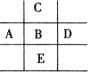
A. PB. MgC. ClD. S
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ�����ʵ���Ũ�ȵ�NaOH��Һʱ�������ĸ�ԭ������������ҺŨ��ƫ��
A.����NaOH�Ѿ�����B.������ƿ�м�ˮδ���̶���
C.������NaOH��Һ�������ձ���D.����ʱ����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и������ӣ��ܴ����������
A.Fe3����I����Cl����Na��B.Cl����K����AlO2����OH��
C.H����Na����K����CO32��D.Ba2����Mg2����HCO3����SO42��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�������������Һ���ò���H2���ǣ� ��
A.Ũ����B.ϡ����C.Ũ����D.ϡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ȼ�����(S2Cl2)�ڹ�ҵ����������Ϊ��ʵ���Һϳ�S2Cl2��ij��ѧ�о���ѧϰС��������й����ϣ��õ�������Ϣ��
�ٽ����﴿����������110�桫140������Ӧ�����ɵ�S2Cl2��Ʒ��
���й����ʵIJ����������±���
���� | �۵�/�� | �е�/�� | ��ѧ���� |
S | 112.8 | 444.6 | �� |
S2Cl2 | ��77 | 137 | �������������S2Cl2��Cl2 |
���ʵ��װ��ͼ���£�
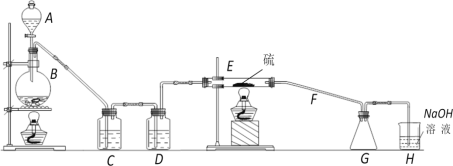
(1)����A��������______________��
B�з�Ӧ�����ӷ���ʽΪ_________________________________��
(2) S2Cl2��ˮ�����ɻ�ɫ���ʡ�һ����ʹƷ����Һ��ɫ�����廯���P������D�е��Լ�Ӧ��________��д���÷�Ӧ�Ļ�ѧ����ʽ__________________ ��
(3)װ��H��������_______________ �����з�����Ӧ�Ļ�ѧ����ʽΪ ______________________��
(4)S2Cl2��Ʒ�п��ܻ��е�������SCl2��Cl2��S,Ϊ�����S2Cl2�Ĵ��ȣ��ؼ��IJ����ǿ��ƺ��¶Ⱥ�_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ������˵����ȷ����
A. ��״���£�22.4L�����������ļ��Թ��ۼ���ĿΪNA
B. 500C��101KPaʱ3.2gSO2��������O2��ַ�Ӧ������SO3����ĿΪ0.05NA
C. 100mL12mol/L��Ũ���������ͭ��Ӧ��ת�Ƶĵ���������0.6NA��0.9NA֮��
D. 1L0.1mol/LNH4Cl��Һ�У�NH4+������Ϊ0.1NA
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com