
����Ŀ����ˮɹ�κ����±�к�Br-������ȡBr2��������ͼ:
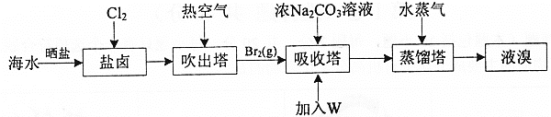
��֪:��3Br2+3CO![]() = 5Br- +BrO
= 5Br- +BrO![]() +3CO2����5Br- +BrO
+3CO2����5Br- +BrO![]() +6H+ =3Br2 +3H2O
+6H+ =3Br2 +3H2O
����˵������ȷ����
A.��ˮɹ����ʵ����Ԫ�صĸ���
B.ͨ��Cl2������Ӧ: 2Br- +Cl2= Br2 +2Cl-
C.�������м���W��Һ��õ�Br2��W����������
D.��ˮ��Br-��Ũ��ԼΪ66mg��L-1 �����ù�������ȡ��Ϊ60%��1m3��ˮ���Ƶ�39.6g Br2
���𰸡�C
��������
A����ˮɹ�κ�õ���±ˮ��Br-��Ũ�ȴ��������ʵ����Ԫ�صĸ�����A��ȷ��
B��ͨ��Cl2���Ŀ�ľ��ǽ�Br-����ΪBr2�ʷ�����Ӧ�����ӷ���ʽΪ��2Br-+Cl2= Br2+2Cl-��B��ȷ��
C���������ǿ�����ԣ�����Br-��Ӧ�����ж��к�������NO���������м���W��Һ��õ�Br2��W�����������ᣬ��Ӧ����ϡ���ᣬ������Ӧ�ڵõ�Br2��C����
D�����������غ��֪����ˮ��Br-��Ũ��ԼΪ66mg��L-1�����ù�������ȡ��Ϊ60%��1m3��ˮ���Ƶ�66mg��L-1��1000L��60%��10-3g/mg=39.6g Br2��D��ȷ��
�ʴ�Ϊ��C��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ�鷽�����ܴﵽʵ��Ŀ����
ʵ��Ŀ�� | ʵ�鷽�� | |
A | ֤�������鷢����ȥ��Ӧ����ϩ���� | ���Թ��м����������������NaOH���Ҵ���Һ�����ȣ���������������ͨ��������Ȼ�̼��Һ |
B | ����±������±ԭ�ӵ����� | ��������������������Һ���ȣ�ȡ��ȴ��ӦҺ�������еμ������ữ�����������Һ |
C | ���� | �� |
D | ��֤����Һ���� | ����Ӧ�����Ļ��������ͨ�����Ȼ�̼��Һ��ͨ�� |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���A�������л��ϳɵ��м��壬������ͼ��������Է�������Ϊ84����֪16.8 g A��ȫȼ������44.0 g CO2��14.4 g H2O����ش��������⣺
(1)A�ķ���ʽ��____________��
(2)���������������A�����к����ǻ���λ�ڷ���һ�˵�C��C�����Һ˴Ź����������������壬�����֮��Ϊ6��1��1����A�Ľṹ��ʽ��_______��
(3)�л���B��A��ͬ���칹�壬1 mol B����1 mol Br2�ӳɡ����л���������̼ԭ����ͬһ��ƽ���ϣ�û��˳���칹����д��B������������ͭ��Ӧ�Ļ�ѧ����ʽΪ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ��Ni�ĵ�������λ�����������ӿռ�ṹΪ�����塣1mol��������ϡˮ��Һ������![]() �������
�������![]() ����233g������Һ���ˣ���Һ����������Һ��ϲ����ɳ�����Ԫ�ط����������������������ԭ�Ӹ�����Ϊ
����233g������Һ���ˣ���Һ����������Һ��ϲ����ɳ�����Ԫ�ط����������������������ԭ�Ӹ�����Ϊ![]() ��
��
��1���뻭��Ni�ļ۵����Ų�ͼ��______________________��H��N��O�ĵ縺���ɴ�С��˳��Ϊ______________________��
��2����Һ����������Һ��ϲ����ɳ�����ԭ����___________________________________��
��3��������������ӵ�����ԭ���ӻ���ʽΪ____________����д���������Ļ�ѧʽ��________________��
��4����ԭ�ӷ����и�ԭ������ͬһƽ�棬�����ƽ�е�p�������p���ӿ����ڶ��ԭ�Ӽ��˶����γ�������![]() �������������д���������
���������������������![]() ��������_________
��������_________![]() �����
�����![]() ��
��
A.![]()
![]()
![]()
![]() ��
��
��5����ͼ������NiAs�����ľ���ͼ���������������![]() ��
��![]() ��
��![]() ���������㡢����С��ΪNi��As�����ڡ�
���������㡢����С��ΪNi��As�����ڡ�
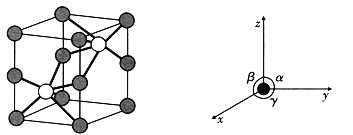
��д��As����λ��Ϊ_________��Ni����λ��Ϊ_________��
����֪��������Ϊ![]() ��
��![]() �������ܶ�Ϊ____________
�������ܶ�Ϊ____________![]() д����ʽ
д����ʽ![]() ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ����1��18��Ԫ���в���Ԫ����ɵ�һЩ���ʼ��仯����֮���ת����ϵͼ�����³�ѹ�£�D��F��K��Ϊ��ɫ�̼�����ζ�����壬B���������ɫҺ�壬A���ɵ���C��D��ȼ�����ɵĵ���ɫ���塣����Ӧ�����ɵIJ�����������ȥ��
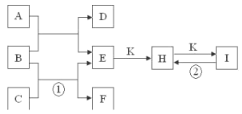
��ش��������⣺
��1������A�Ļ�ѧʽΪ_______��
��2��������E����ˮ�еĵ��뷽��ʽΪ_________________________________��
��3��ʵ���ҽ�����Cͨ��������_______�У���д����Ӧ�ٵ����ӷ���ʽ�����õ����ű������ת�Ƶķ������Ŀ_____________________________��
��4����Ӧ�ڵĻ�ѧ����ʽΪ____________________��
��5���������ֱ�װ��H��I������Լ�ƿ�����ǩ����������֡�������������ǣ������Լ����п�ѡ�õ���________.
A.����ʯ��ˮ B.ϡ���� C.���ȣ����� D.��ɫ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ķ����п��ն��ģ��ش����⡣
��������ί�����ġ�����״�����������Ʒ���(���С����ᵽ:����Է�ջ����У���ʱ�䱩¶�ڸ�Ũ�����ܽ�����£��¹ڲ������ھ����ܽ������Ŀ��ܡ����ҽ����ƿ��ֿ��������ã����в����ķ�ĭ�ᱻ�����м�������IJ�(��Ҫ�������۱�ϩ)����������Ҳ�ǿ��Ʋ�����������Ч��ʩ��������״�������������ڼ�Ԥ��������ָ��������:����������ѡ������������__%�ƾ���ʳ������Ҫ�������15�������ϡ�
(1)���ܽ������ӵĴ�СΪ_______��
a��< lnm b��> 100nm c��1nm~100nm
(2)��ϩ�ڴ����������Ʊ��۱�ϩ����ѧ��Ӧ����ʽΪ_______________
(3) 75%�ƾ�����Ч�ɷ����Ҵ����Ҵ���һ��ͬ���칹��Ľṹ��ʽΪ__________��
(4)ʳ���߿�ͨ����������������������ڼ��������·�����______��
(5)����������һ�ֵ���������� ����������������ȴ��Ҵ���������������Ӧ:
i��CH2=CH2+Cl2+H2O ��ClCH2CH2OH+HCl
ii��ClCH2CH2OH+ HCl+Ca(OH)2 �� ![]() + CaCl2 +2H2O
+ CaCl2 +2H2O
�ִ�ʯ�ͻ������õ��´���: 2CH2=CH2+O2![]()
![]()
���ȴ��Ҵ�����ȣ����´������ŵ���________��_________�� ( ������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijУ̽����ѧϰС�����Ѳ�������ķ���м����ӡˢ��·��ĸ�ʴ����������ͭ��̽���������£�
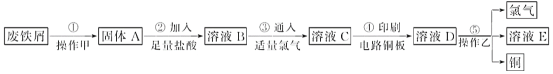
��ش��������⣺
(1)����ҺB��ֻ����Fe2��������Fe3������֤������ʵ��ʵ�鷽����________(�����)��
A���ȼ���ˮ�����KSCN��Һ���Ժ�ɫ
B���ȼ�KSCN��Һ�����Ժ�ɫ���ټ���ˮ���Ժ�ɫ
C����ֻ�μ�KSCN��Һ���Ժ�ɫ
(2)����ܵĻ�ѧ����ʽΪ_____________________________��
(3)����ȥ�Ȼ��������Ȼ������Լ����Լ�________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʹ�������ǿ����������ܵ���Ҫ�о�����
(1)![]() ��һ�ִ�����ϣ�����
��һ�ִ�����ϣ�����![]() ��
��![]() ��Ӧ�Ƶá�
��Ӧ�Ƶá�
����̬Clԭ���У�����ռ�ݵ���ߵ��Ӳ����Ϊ ______���õ��Ӳ���е�ԭ�ӹ����Ϊ _______��
��Li��B��HԪ�صĵ縺���ɴ�С������˳��Ϊ ___________��
(2)�����⻯���Ǿ������÷�չǰ���Ĵ�����ϡ�
��LiH�У����Ӱ뾶��Li+ ___________(����>����=������<��)H-��
��ij��������Ƕ����ڽ���Ԫ��M���⻯�M�IJ��ֵ����������ʾ��
|
|
|
|
|
738 | 1451 | 7733 | 10540 | 13630 |
��M�� ______________ (��Ԫ������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
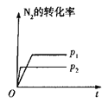
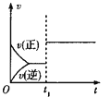
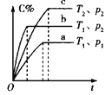
����Ŀ�����ж�ͼ���������ȷ����

�� �� �� ��
A.ͼ�ɱ�ʾѹǿ�Է�Ӧ��![]()
![]() ��Ӱ��
��Ӱ��
B.ͼ���У�![]() ʱ�̸ı������һ���Ǽ����˴���
ʱ�̸ı������һ���Ǽ����˴���
C.��ͼ����ʾ��Ӧ��![]() ����
����![]() ��
��![]()
D.ͼ����ʾˮ��![]() ��
��![]() �Ĺ�ϵ��ABC������������
�Ĺ�ϵ��ABC������������![]()
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com