
����Ŀ����ԭ���������������ֶ�����Ԫ�أ�����ĸx�ȱ�ʾ��ԭ�Ӱ뾶����Դ�С��������ۻ�����۵ı仯����ͼ��ʾ��

�����жϳ���Ԫ�ػش����⣺
����Ԫ�ؿ������R��zx4f(gd4)2����ʢ��10mL 1mol��L��1R��Һ���ձ��еμ�1mol��L��1NaOH��Һ���������ʵ�����NaOH��Һ����ı仯ʾ��ͼ���£�

(1)R��Һ�У�����Ũ���ɴ�С��˳����___________________________________
(2)д��m�㷴Ӧ�����ӷ���ʽ��__________________________________________��
(3)����R��Һ�иļ�20mL 1.2mol��L��1 Ba(OH)2��Һ����ַ�Ӧ����Һ�в������������ʵ���Ϊ________ mol��
���𰸡�c(SO42-)��c(NH4+)��c(Al3��)��c(H��)��c(OH��)NH4+��OH��===NH3��H2O0.022
��������
��ͼ�еĻ��ϼۺ�ԭ�Ӱ뾶�Ĵ�С����֪x��HԪ�أ�y��CԪ�أ�z��NԪ�أ�d��OԪ�أ�e��NaԪ�أ�f��AlԪ�أ�g��SԪ�أ�h��ClԪ�ء�
(1)��NH4Al(SO4)2����Һ�з������룺NH4Al(SO4)2=NH4++Al3++2SO42-��NH4+��Al3+����ˮ�ⷴӦ������ˮ���������OH-��ʹ��Һ��c(H+)����������Һ�ﵽƽ��ʱ��c(H+)��c(OH-)����Һ�����ԣ�����Al3+ˮ��̶ȴ���NH4+�����c(NH4+)��c(Al3+)����ˮ��̶������ģ���Ҫ���ε�����������Ӵ��ڣ������Һ������Ũ�ȴ�С��ϵ�ǣ�c(SO42-)��c(NH4+)��c(Al3+)��c(H+)��c(OH-)���ʴ�Ϊ��c(SO42-)��c(NH4+)��c(Al3+)��c(H+)��c(OH-)��
(2)m������м����������ƣ��������ʵ������䣬��NH4+��OH-��Ӧ����NH3H2O�����ӷ���ʽΪNH4++OH-=NH3H2O���ʴ�Ϊ��NH4++OH-=NH3H2O��
(3)10mL 1molL-1 NH4Al(SO4)2��Һ��Al3+ ���ʵ���Ϊ0.01mol��NH4+�����ʵ���Ϊ0.01mol��SO42-�����ʵ���Ϊ0.02mol��20mL 1.2 molL-1Ba(OH)2��Һ��Ba2+���ʵ���Ϊ0.024mol��OH-Ϊ0.048mol����SO42-+Ba2+=BaSO4������֪SO42-���㣬�ʿ��Եõ�0.02mol BaSO4��
Al3+ + 3OH- = Al(OH)3��
0.01mol 0.03mol 0.01mol
��Ӧʣ��OH-Ϊ0.048mol-0.03mol=0.018mol��
NH4+ + OH- = NH3H2O
0.01mol 0.01mol
��Ӧʣ��OH-Ϊ0.018mol-0.01mol=0.008mol��
Al(OH)3 + OH-=AlO2-+2H2O
0.008mol 0.008mol
�ʵõ�Al(OH)3����Ϊ0.01mol-0.008mol=0.002mol�������յõ�����Ϊ0.02mol+0.002mol=0.022mol���ʴ�Ϊ��0.022��


 �ŵ������ϵ�д�
�ŵ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʹһþ���Ͻ��ĩ�ڹ���ϡH2SO4���ܽ⣬��������Һ�м���NaOH��Һ�����ɳ���������W�ͼ���NaOH��Һ�����V�Ĺ�ϵ��ͼ��ʾ����úϽ���þ���������ʵ���֮��Ϊ

A. 1��1 B. 2��3 C. 8��9 D. 4��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ǰ������ԭ���������������Ԫ��A��B��C��D��E�У�A�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������ȣ�Bԭ�Ӻ��������7�ֲ�ͬ���˶�״̬��CԪ��ԭ�Ӻ����M����ֻ��2�ԳɶԵ��ӣ�D����C2���ĵ�������ȣ�EԪ��λ��Ԫ�����ڱ���ds�����һ�̬ԭ�Ӽ۵����Ӿ��ѳɶԡ�
�ش��������⣺
(1)E2������Χ�����Ų�ͼΪ________________��
(2)����Ԫ���е縺����С����________(��Ԫ�ط���)��CAB�������У�Aԭ�ӵ��ӻ���ʽ��________��
(3)AB����D����E2������������ɵĻ�ѧ����D2E(AB)4���û������д���һ���������ӣ������ӵĻ�ѧʽΪ________����λ����________��
(4)C��E����Ԫ����ɵ�һ�ֻ�����ľ�����ͼ��ʾ��
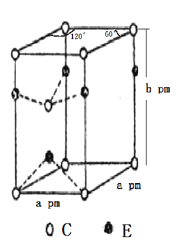
�ٸû�����Ļ�ѧʽΪ________��E����λ��Ϊ________��C��ȡ________(����������������������������������ܡ��������������ܡ�)�ѻ���
����ʽ��ʾ�þ�����ܶȣ�________g��cm��3��(NA��ʾ�����ӵ�������ֵ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����ﳣ��Ũ������������̷�Ӧ����ȡ��������������Ӧ�Ļ�ѧ����ʽΪ��MnO2��4HCl(Ũ) ![]() MnCl2��Cl2����2H2O��ȡһ������Ũ����ʹ����������̷�����Ӧ�������������ڱ�״���µ����Ϊ22.4 L��
MnCl2��Cl2����2H2O��ȡһ������Ũ����ʹ����������̷�����Ӧ�������������ڱ�״���µ����Ϊ22.4 L��
��ش��������⣺
(1)�μӷ�Ӧ�Ķ������̵�����Ϊ____________��
(2)��Ӧ�б�������HCl�����ʵ���Ϊ ____________��
(3)ʵ���ұ��õ�Ũ������������Ϊ36.5%���ܶ�Ϊ1.19g��cm��3����������Ũ��������ʵ���Ũ��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����̬Gaԭ����������ߵ��ܲ����________ ��______ �ֲ�ͬ�����ĵ���,GaԪ����ͬ��������Ԫ��Zn��Ge��ȣ���һ�����ܴӴ�С��˳��__________,����Ԫ�ط��ű�ʾ������Zn��������������ܶѻ��������ò�ѻ���ʽΪ__________ ��ABABAB��ABCABC�����־���ͷǾ�����ɿ��Ŀ�ѧ������________________��
��2���������ֺ����� H3PO2 �� H3PO3 �� H3PO4 ������ԭ�Ӿ���sp3�ӻ�������ԭ���γ��ĸ��� �������H3PO3�Ľṹʽ��__________����д��H3PO2 ������ǿ��������Һ��Ӧ�Ļ�ѧ����ʽ_________�����������ǿ��˳��ΪH3PO2 <H3PO3 < H3PO4����ԭ����___________����HNO3 �� HNO2 ����ԭ�ӵ��ӻ���ʽ�ֱ�Ϊ__________��
��3������ͭͶ�백ˮ�����������Һ�о�����������Ͷ�백ˮ��������Ļ����Һ�У���ͭƬ�ܽ⣬��Һ������ɫ����д���÷�Ӧ�����ӷ�Ӧ����ʽ____________________________ ������֪NF3��NH3�Ŀռ乹�Ͷ��������Σ���NF3������Cu2+�γ������ӣ�ԭ����_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�����ô�ZnO(��FeO��CuO)��ȡ����п���������£����ֲ���ʡ�ԣ���

��֪�����������γ������������ʱ��pH���±���
������������ | Fe2�� | Fe3�� | Zn2�� | Cu2�� |
��ʼ����ʱpH | 6.3 | 1.5 | 6.2 | 5.2 |
������ȫʱpH | 9.7 | 3.2 | 8.0 | 6.4 |
��ش��������⣺
(1)���������п�Ĺ����У�Ϊ�˼ӿ�������ʣ��ɲ�ȡ�Ĵ�ʩ��______________ (д��һ�㼴��)��
(2)����A��Ŀ���ǽ�Fe2������ΪFe3������ȫ���γ�Fe(OH)3������Ϊ���ݲ��γ�Cu(OH)2��Zn(OH)2���ò����������ҺpH�ķ�Χ��______________���ò����м���H2O2������Ӧ�����ӷ���ʽΪ_______________________________________��
(3)����D�IJ�������Ϊ________________________________________________��
(4)�ɴ�ZnO��ȡ����п����һ�ַ����ǽ���ZnO����FeO��CuO������NaOH��Һ��ZnOȫ
��ת��ΪNa2[Zn(OH)4]��Һ���÷�Ӧ�Ļ�ѧ����ʽΪ_________________________��Ȼ��FeO��CuO���˳�ȥ�����ö��Ե缫������Һ���������ݳ���ɫ��ζ���壬����������п���������缫��ӦʽΪ_____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ����С���ʵ����ȡ�������Լ����Լ�ƿ�ϱ�ǩ�IJ���������ͼ��ʾ����С��������450 mL 0.2 mol��L��1��ϡH2SO4��Һ��

(1)�����Լ�ƿ��������Һ�����Ϊ________mL��
(2)������Ͳ������ƿ�Ĺ��Ϊ________��________��
(3)Ũ����ϡ��ʱ�IJ�����________________________��
(4)����Ϊ��С��IJ��������ܵ���������ҺŨ��ƫ�ߵ���________��
A����Һǰδ��ȴ������
B������ʱ��ˮ���ˣ��õι�����
C������ʱ���ӿ̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʯ��ʯ���Կ���CaO��FeO��Fe2O3��Al2O3��SiO2��ɡ���ҵ�϶�������ۺ����õ��������£�
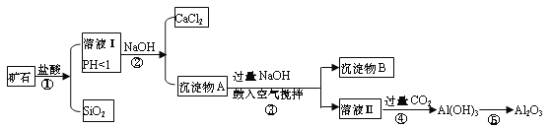
��1���÷���ʽ��ʾʢ������������Һ���Լ�ƿ�����ò�������ԭ��________��
��2����Һ���г���Ca2+�⣬�����ܺ��еĽ�����������_______________��
��3���������NaOH�μӷ�Ӧ�����ӷ���ʽ��________________�������������ֽ����Ŀ����_____________________��
��4���ڹ�ҵ�����У������ͨ�����CO2�������������ԭ����___________��д�������ͨ�����CO2�����ӷ���ʽ _________________________________��
��5�������ʵ��֤����ʯ���к���FeO���Լ���ѡ��˵��ʵ�����������_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��һ��̼ԭ����������ǻ�ʱ���ȶ���
������A����ͼ��ת����ϵ��
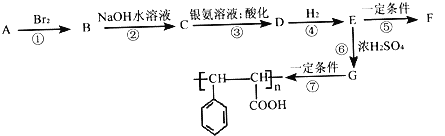
��1����֪1molA����2molBr2�ӳɣ�д��A�Ľṹ��ʽ___________________��д��B�ķ���ʽ___________________��
��2��D�к��������ŵ�����Ϊ______________��ָ����Ӧ�ķ�Ӧ���ͣ�___________________��
��3��д��C��������Һ��Ӧ�Ļ�ѧ����ʽ��_________________________________��
��4��E�ж���ͬ���칹�壬�������������Ĺ���__________�֡�
�����ڷ��㻯��� ����ʹFeCl3��Һ������ɫ��Ӧ��
�ۺ������Ľṹ�� �ܱ�����������ȡ������
���к˴Ź�����������6�����շ壬�����֮��Ϊ1��2��2��2��2��1���л���ṹ��ʽΪ___________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com