
����Ŀ������˵����ȷ����( )
A.NA���������ӵ�������1mol C4H10�к����ۼ���ĿΪ14NA
B.ij��Ӧ����H = ��88kJmol-1��������Ӧ���һ��С��88kJmol-1
C.��֪ij�¶��£�Kw = 1��10-13������pH =8��NaOH��Һ��pH =5��H2SO4��Һ��ϱ����¶Ȳ��䣬��ʹ�����ҺpH =7����NaOH��Һ��H2SO4��Һ�������Ϊ11��9
D.��Ũ��Ϊ0.1molL-1 HF��Һ��ˮ����ϡ�����У�����Ⱥ�Ka(HF)���ֲ��䣬![]() ʼ�ձ�������
ʼ�ձ�������
���𰸡�C
��������
A. 1mol C4H10�к�3��C��C��10��C��H���ۼ����ۼ���ĿΪ13NA����A����
B. �ʱ�Ĵ�С���������淴Ӧ�Ļ�ܵIJ�ֵ��ij��Ӧ����H = ��88kJmol-1��������Ӧ��ܲ�һ��С��88kJmol-1����B����
C. ˮ�����ӻ�����Ϊ1��10-13��![]() ��pH =6.5ʱ����Һ�����ԣ�pH =5��H2SO4��Һ��
��pH =6.5ʱ����Һ�����ԣ�pH =5��H2SO4��Һ��![]() ��pH =8��NaOH��Һ������
��pH =8��NaOH��Һ������![]() ��
��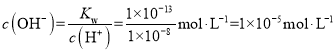 ��Ҫʹ���Һ��pH =7����Һ�ʼ��ԣ������������ӹ����������Һ�����������ӵ����ʵ���Ũ��
��Ҫʹ���Һ��pH =7����Һ�ʼ��ԣ������������ӹ����������Һ�����������ӵ����ʵ���Ũ��![]() ����������Һ��NaOH��Һ���������ΪX��Y����������Һ��Ϻ����OH-���ʵ���Ũ��Ϊ
����������Һ��NaOH��Һ���������ΪX��Y����������Һ��Ϻ����OH-���ʵ���Ũ��Ϊ![]() ������Y��X = 11:9����C��ȷ��
������Y��X = 11:9����C��ȷ��
D. ��Ũ��Ϊ0.1molL-1 HF��Һ��ˮ����ϡ�����У����������Ka(HF)���ֲ��䣬c��H+����c��F-����С��c��OH-��������Һ�еĵ���غ�c(H+)=c(F-)+c(OH-)��![]() =
=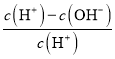 =1-
=1-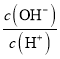 ������D����
������D����
������������ΪC��


 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������ȼ�շ��ⶨij�ְ�����(CxHyOzNm)�ķ�����ɡ�ȡWg���ְ�������ڴ����г��ȼ�գ����ɶ�����̼��ˮ�͵���������ͼ��ʾװ�ý���ʵ�顣

�ش��������⣺
��1��ʵ�鿪ʼʱ������ͨ��һ��ʱ�����������������__________________��
��2������װ������Ҫ���ȵ�������_______ (��д��ĸ)������ʱӦ�ȵ�ȼ_____���ľƾ��ơ�
��3��Aװ���з�����Ӧ�Ļ�ѧ����ʽ��____________________________��
��4��Dװ�õ�������____________________________��
��5����ȡ���������ʱ��Ӧע����_________________����_________________��
��6��ʵ���в�õ��������ΪVmL(��״��)��Ϊȷ���˰�����ķ���ʽ������Ҫ���й�������____________________��
A�����ɶ�����̼���������
B������ˮ������
C��ͨ�����������
D�����������Է�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������洦����Ƥ�����ơ�ӡȾ�ȶ�������ɸ���Ⱦ�����۸������۸����Ըߣ����ױ����������������������
����ҵ�ϴ������Ժ�Cr2O72����ˮ�ķ������£�
����Cr2O72�������Է�ˮ�м���FeSO4��Һ��ʹCr2O72��ȫ��ת��ΪCr3����д���÷�Ӧ�����ӷ���ʽ��_____��
��������Һ��pH��ʹCr3����ȫ������ʵ���Ҵ��Բⶨ��ҺpH�ķ���Ϊ_____��25������������Һ��pH=8������Һ�в���Cr3�������ʵ���Ũ��Ϊ_____mol/L������֪25��ʱ��Ksp[Cr(OH)3]=6.3��10��31��
����Ԫ����Ũ�ȵIJⶨ��ȷ��ȡ25.00mL��Cr2O72����Cr3�������Է�ˮ�������м���������(NH4)2S2O8��Һ��Cr3��������Cr2O72������г�ȥ������(NH4)2S2O8����������Һ�м��������KI��Һ����ַ�Ӧ���Ե���Ϊָʾ���������еμ�0.015mol/L��Na2S2O3����Һ���յ�ʱ����Na2S2O3��Һ20.00mL��
��֪�ⶨ�����з����ķ�Ӧ���£�
��2Cr3����3S2O82����7H2O =Cr2O72����6SO42����14H��
��Cr2O72����6I����14H��=2Cr3����3I2��7H2O
��I2��2S2O32��=2I����S4O62��
�����ˮ�и�Ԫ����Ũ�ȣ���λ��mg��L��1��д��������̣���_____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2019��ŵ������ѧ������Լ�����ŵ����ɷ�˹̹������͢��ķ�ͼ�Ұ����λ��ѧ�ң��Ա���������﮵�������������ľ��ס���ش��������⣺
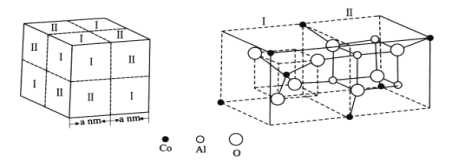
��1��LiCoO2��LiFePO4����������ӵ�ص��������ϡ���̬Coԭ�Ӻ�������Ų�ʽΪ___����̬��ԭ���У�����ռ�ݵ�����ܲ����Ϊ___�����ܲ�������ߵĵ������ڿռ���___����չ����ԭ�ӹ����___�Ρ�
��2��[Co(NO3��)4]2����Co2+����λ��Ϊ4��������N���ӻ���ʽΪ__�����������и�Ԫ�صĵ�һ��������С�����˳��Ϊ___(��Ԫ�ط���)��1mol���������к��Ҽ���ĿΪ___NA��
��3��LiFePO4���ڼ������Σ���ֱ���Ķ�����������һ�ָ��������Σ��磺�������ơ��������Ƶȡ�����������ӡ��������������ͼ��ʾ��
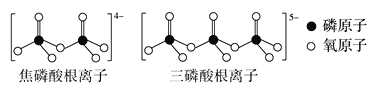
������������ӵĻ�ѧʽ����ͨʽ��ʾΪ___(��n����Pԭ����)��
��4����������ṹ��ͼ��������������4��I�ͺ�4������С�����幹�ɣ��仯ѧʽΪ___��������Al3+ռ��O2���γɵ�___(��������϶���������϶��)��NAΪ�����ӵ�������ֵ������������ܶ�Ϊ___g��cm��3(�м���ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л��������ұ�(GB2760-2011)�涨���Ѿ���SO2���ʹ����Ϊ0.25 g��L-1��ij��ȤС����ͼ1װ��(�г�װ����)�ռ�ij���Ѿ���SO2�������京�����вⶨ��


��1������A��������_________��ˮͨ��A�Ľ���Ϊ_________��
��2��B�м���300.00 mL���Ѿƺ��������ᣬ����ʹSO2ȫ���ݳ�����C��H2O2��ȫ��Ӧ���仯ѧ����ʽΪ______________________________________________________��
��3����ȥC�й�����H2O2��Ȼ����0.090 0 mol��L-1NaOH����Һ���еζ����ζ�ǰ������ʱ��Ӧѡ��ͼ2�е�_________�����ζ��յ�ʱ��Һ��pH��8.8����ѡ���ָʾ��Ϊ_________������50 mL�ζ��ܽ���ʵ�飬���ζ����е�Һ���ڿ̶���10�����������Һ������(�����)_________(����10 mL������40 mL����<10 mL����>40 mL)��
��4���ζ����յ�ʱ������NaOH��Һ25.00 mL�������Ѿ���SO2����Ϊ_________g��L-1��
��5���òⶨ�����ʵ��ֵƫ�ߣ�����ԭ����������װ������Ľ���ʩ��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±���Ԫ�����ڱ���һ���֣����ݱ��и�����10��Ԫ�أ��ش��������⡣

(1)��������ǿ��Ԫ����_________����ѧ��������õĵ�����___________��
(2)Ca������������Ϊ____________________��
(3)C��O�У�ԭ�Ӱ뾶�ϴ����_____________��
(4)ij���ӵ�ԭ�ӽṹʾ��ͼΪ![]() �����Ӧ�����ӷ�����___________��
�����Ӧ�����ӷ�����___________��
(5)����������Ҫ������ֱ�ΪSO2��___________(�ѧʽ)��
(6)H2S��HCl�У����ȶ��Խ�ǿ����___________��
(7)Si�Ǵ���������������Ҫ����֮һ����������Ҫ��;Ϊ��___________(д������һ��)����������Ļ�ѧʽΪ��___________��
(8)�ơ�������������ˮ����֮�䷴Ӧ�Ļ�ѧ����ʽ��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ����
A. 95��ʱ��ˮ��pH<7��˵�����ȿɵ���ˮ������
B. pH=3�Ĵ�����Һ��ϡ����10��ʱ��Һ��pH<4
C. ��Ũ�ȵĴ�����Һ������������Һ�������Ϻ�pH=7
D. �����pH��Ϊ3�Ĵ��������ֱ�������Zn��Ӧ�����������H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
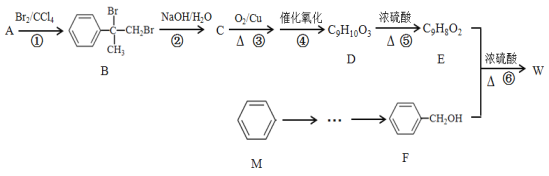
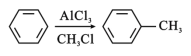
����Ŀ���л���W��C16H14O2��������������߷��Ӳ��Ϻϳɵ��м���ȣ��Ʊ�W��һ�ֺϳ�·�����£�
��֪��
��ش��������⣺
��1��F�Ļ�ѧ������_______���ݵķ�Ӧ������_______��
��2��E�к��еĹ�������_______��д���ƣ���E��һ�������¾ۺ����ɸ߷��ӻ�����ø߷��ӻ�����Ľṹ��ʽΪ_______��
��3��E + F��W��Ӧ�Ļ�ѧ����ʽΪ_______��
��4����A������ͬ�������Һ��б�����ͬ���칹�廹��_______�֣����������칹�������к˴Ź�������Ϊ����壬�ҷ����֮��Ϊ1��1��2��2��2��2�Ľṹ��ʽΪ____��
��5�������л���W�������ϳ�·�ߣ�д����M��CH3ClΪԭ���Ʊ�F�ĺϳ�·�ߣ����Լ���ѡ��_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��ͨ��5min����������2.16g��ͬʱ��A�����ռ�����״���µ�����224mL��A���л��Һ�����Ϊ200mL�����ǰ�������Ϊ���䣩����ͨ��ǰA����ԭ�����ҺCu2+��Ũ�ȡ�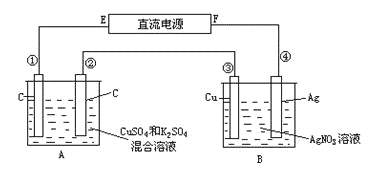
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com