
����Ŀ��ij�¶��£���Ӧ2A��g��![]() B��g��+C��g����ƽ�ⳣ��Ϊ1�����ݻ�Ϊ2 L���ܱ������м���A��g����20 sʱ��ø���ֵ����ʵ������±���
B��g��+C��g����ƽ�ⳣ��Ϊ1�����ݻ�Ϊ2 L���ܱ������м���A��g����20 sʱ��ø���ֵ����ʵ������±���
�� �� | A��g�� | B��g�� | C��g�� |
���ʵ���/mol | 1.2 | 0.6 | 0.6 |
����˵����ȷ���ǣ� ��
A. ��Ӧǰ20 s��ƽ������Ϊv��A��=0.6 mol��L-1��s-1
B. 20 sʱ������Ӧ���ʵ����淴Ӧ����
C. ��ƽ��ʱ��A��g����ת����Ϊ100%
D. �������¶ȣ�ƽ�ⳣ����Ϊ0.6����Ӧ����H��0
 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ���������Һ����ԶԷ�Ӧ���ʵ�Ӱ�졣�ڳ����°������·������ʵ�顣
ʵ���� | ��Ӧ�� | ���� |
�� | 10mL2%H2O2��Һ | �� |
�� | 10mL5%H2O2��Һ | �� |
�� | 10mL5%H2O2��Һ | 1mL0.1mol��L��1FeCl3��Һ |
�� | 10mL5%H2O2��Һ������HCl��Һ | 1mL0.1mol��L��1FeCl3��Һ |
�� | 10mL5%H2O2��Һ������NaOH��Һ | 1mL0.1mol��L��1FeCl3��Һ |
��1��ʵ��ٺ͢ڵ�Ŀ����___��
��2��ʵ��ۢܢ��У�������������������ʱ��仯�Ĺ�ϵ��ͼ1������ͼ1�ܹ��ó���ʵ�������__��

��3������0.1gMnO2��ĩ��50mLH2O2��Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ2��ʾ����Ӧ���ʱ仯��ԭ����__��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ���׳ص��ܷ�ӦʽΪ��N2H4+O2=N2+H2O�����й��ڸõ�ع���ʱ��˵����ȷ������ ��
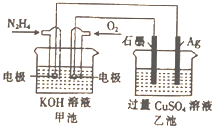
A. ��װ�ù���ʱ��Ag�缫������������
B. �׳��и�����ӦΪN2H4-4e-=N2+4H+
C. �׳غ��ҳ��е���Һ��pH����С
D. ���׳�������0.1molN2H4ʱ���ҳ���������������6.4g����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������и������գ�
(1)�⻯��(NaH)����ʽΪ______���⸺���ӵ����ӽṹʾ��ͼΪ______��
(2)Ԫ��X�������������Ǵ�����������2��,��Ԫ��������___����Ԫ�غ�������Ų�ʽΪ______��������Ԫ�ص���Χ�����Ų�ͼ______��
(3)ǰ������Ԫ����,δ�ɶԵ�����Ϊ5��Ԫ�ط�����___ ,��Ԫ�������ڱ��е�λ��Ϊ��__����___��, ___����
(4)C��N��O�ĵ�һ�������ɴ�С��˳��Ϊ______����������Ԫ�غ���ԭ�ӹ�ͬ��ɵ����ӻ�����Ļ�ѧʽΪ______��
(5)�����������(SeO32-)��VSEPRģ��Ϊ______��������ԭ�ӵĹ���ӻ���ʽΪ______��
(6)������ͭ��Һ����μ��백ˮ���õ���ɫ����Һ��,�ټ��뼫�Խ�С���ܼ��Ҵ�,��������ɫ����![]() ,�þ�����������Ϊ____ ,�������е���λ��Ϊ___ ,��λ��Ϊ___��
,�þ�����������Ϊ____ ,�������е���λ��Ϊ___ ,��λ��Ϊ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����úΪ��Ҫԭ�Ͽ����Ʊ��Ҷ�������ع����������£�

��1��д������l�ڴ�����������ֱ����ȡ�Ҷ����Ļ�ѧ����ʽ_______
��2���ϳ����ڲ�ͬ���������£����Ժϳɲ�ͬ�����ʡ��������ʽ��úϳ���Ϊԭ�Ͼ��ܵõ���ԭ��������Ϊ100%����_____����ĸ����
A������( HOOC-COOH) B���״�(CH3OH) C������[CO(NH2)2]
��3����ҵ�ϻ�����������Ȼ������Ҫ�ɷ�ΪCH4����C02��Ӧ�Ʊ��ϳ�������֪��
CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H=-890.3kJ/mol
2H2(g)+ O2(g)= 2H2O(l) ��H=-571.6kJ/mol
2CO(g)+O2(g)=2CO2(g) ��H=-566.0kJ/mol
��CH4��CO2���ɺϳ������Ȼ�ѧ����ʽΪ____________________��
��4������2���ں����ܱ�������Ͷ������������H2�������·�Ӧ��
CH3OOC��COOCH3(g)+ 4H2(g)![]() HOCH2CH2OH(g)+2CH3OH(g)��H=-34kJ/mol
HOCH2CH2OH(g)+2CH3OH(g)��H=-34kJ/mol
Ϊ����Ҷ����IJ��������ʣ��˲��õĴ�ʩ��___________(����ĸ)��
A�������¶� B������ѹǿ C����������Ũ��
��5�����������ˮ�����ɲ��CH3OOC��COOCH3+ 2H2O![]() HOOC��COOH+2CH3OH
HOOC��COOH+2CH3OH
�ٲ����Ƕ�Ԫ���ᣬ�����Ʊ�![]() ��������أ���
��������أ���![]() ��Һ�����ԣ��û�ѧƽ��ԭ�����ͣ�__________________��
��Һ�����ԣ��û�ѧƽ��ԭ�����ͣ�__________________��
����һ����![]() ��Һ�еμ�NaOH��Һ�����ԡ����й�ϵһ������ȷ����_______������ĸ����
��Һ�еμ�NaOH��Һ�����ԡ����й�ϵһ������ȷ����_______������ĸ����
A��![]()
B��![]()
C��![]()
��6���Ҷ�����������KOH��Һ�й���ȼ�ϵ�أ������Ҷ����ĵ缫Ϊ��Դ��_____����������������������������ӦʽΪ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ҫ�ɷ���![]() ����
����![]() ��
��![]() ��MgO�����ʣ�����ȡ���ֹ���Ʒ���������£�
��MgO�����ʣ�����ȡ���ֹ���Ʒ���������£�
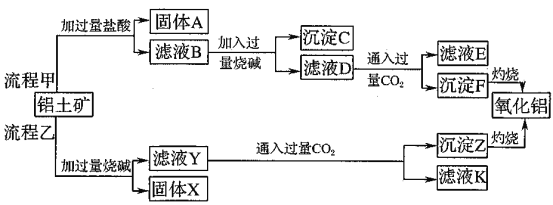
��ش��������⣺
��1�����̼������������Al3+�ķ���ʽΪ_________.
��2�������Ҽ����ռ������SiO32-�����ӷ���ʽΪ________.
��3����֤��ҺB��![]() ����ȡ������Һ������________�����Լ����ƣ���
����ȡ������Һ������________�����Լ����ƣ���
��4����ҺE��K�����ʵ���Ҫ�ɷ���________(�ѧʽ)��д������Һ��һ����;________
��5����֪298Kʱ��![]() ���ݶȻ�����
���ݶȻ�����![]() =5.6��
=5.6��![]() ,ȡ��������ҺB,����һ�������ռ�ﵽ������Һƽ�⣬���PH=13.00������¶��²�������Һ�е�
,ȡ��������ҺB,����һ�������ռ�ﵽ������Һƽ�⣬���PH=13.00������¶��²�������Һ�е�![]() =_______.
=_______.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ�����ʮһ�����˷ɴ��ھ�Ȫ���Ƿ������ijɹ����䣬Ϊ���Ǹ��õ����տռ佻��ԽӼ�������չ����۲��춨�˻������ҹ����캽��ɴ�����Ҫ���������������Ҳ����Ϊ��ɵĽ���������������ʻش��������⣺
(1)���ڹ�ҵұ�����Ļ�ѧ����ʽΪ _______________��
(2)����γɻ�����LiAlH4���ǽ���������������л��ϳ��еij����Լ�����ˮ�ܵõ���ɫ��Һ�����ҷֽ��ͷų�H2��LiAlH4�ڻ�ѧ��Ӧ��ͨ����_______(���������ԭ��)����
(3)�����������Խ�����ִ��������������й㷺��Ӧ�á�
��Al-Ag2O��ؿ�����ˮ�¶�����Դ����ѧ��ӦΪ��2Al+3Ag2O+2NaOH+3H2O��2Na[Al(OH)4]+6Ag�����ĵ缫��ӦʽΪ_______________������������Һ��pH________(�������䡱��С��)��
����һ����������价������ȫ���ܵ�Խ��Խ��Ĺ�ע����ԭ������ͼ��ʾ��
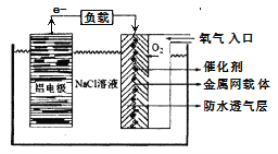
�õ�ص�������Ӧ����ʽΪ_______________�������NaCl��Һ��������_______________���Ըõ��Ϊ��Դ���ö��Ե缫���Na2SO4��Һ����Al�缫��������1.8gʱ�������������ɵ������ڱ�״���µ����Ϊ____________L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ����0.200 0 mol��L��1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ��������£�
��������ˮϴ�Ӽ�ʽ�ζ��ܣ�������ע��NaOH��Һ����0���̶������ϣ�
�ڹ̶��õζ��ܲ�ʹ�ζ��ܼ��촦����Һ�壻
�۵���Һ������0������0���̶������£������¶�����
����ȡ20.00 mL����Һע��ྻ�Ļ�������������ˮ����ƿ�У�������3�η�̪��Һ�� ���ñ�Һ�ζ����յ㣬���µζ���Һ�������
��ش��������⣺
��1�����ϲ����д������______(����)�����ⶨ���ƫ�ߣ���ԭ�������________(����ĸ)��
A.���Ʊ���Һ�Ĺ���NaOH�л���KOH���� B.�ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�����������ȷ C.ʢװδ֪Һ����ƿ������ˮϴ��������δ֪Һ��ϴ D.����ı�NaOH��Һ���ʵ���Ũ��ƫ��
��2���жϵζ��յ��������_____________________________________��
��3����ͼ��ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ________mL��

��4�������������ݣ��������������Ũ�ȣ�________mol��L��1��
�ζ����� | �������(mL) | ���ռ���Һ���(mL) | |
�ζ�ǰ���� | �ζ������ | ||
��һ�� | 20.00 | 0.40 | 20.40 |
�ڶ��� | 20.00 | 2.00 | 24.10 |
������ | 20.00 | 4.00 | 24.00 |
��5����ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�ء�����H2(g)��CO(g)��CH3OH(l)��ȼ������H�ֱ�Ϊ��285.8 kJ��mol��1����283.0 kJ��mol��1�ͣ�726.5 kJ��mol��1����ش��������⣺
����̫���ֽܷ�10 molˮ���ĵ�������________kJ��
��CH3OH(l)����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪ________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com