
����Ŀ��ά���� C����������Ѫ�ᣬ����ʽΪ C6H8O6�����н�ǿ�Ļ�ԭ�ԣ������ڿ������ױ�������������������ͨ������������Һ������֪���ʵ���Ũ�ȵ� I2 ��Һ���вⶨ���÷�Ӧ �Ļ�ѧ����ʽ���£�C6H8O6+ I2 = C6H6O6 +2HI�������ⶨij��Ʒ��ά���� C �������� ��������IJ��輰��õ��������£�ȡ10mL6mol/LCH3COOH���ṩ���Ի�����������100 mL ����ˮ������Һ������к������ȴ����ȷ��ȡ 0.2000g ��Ʒ���ܽ���������ȴ�� ��Һ�У����������ʵ���Ũ��Ϊ 0.05000 mol/L �� I2 ��Һ���з�Ӧ���պ���ȫ��Ӧʱ������21.00 mL I2 ��Һ��
��1��CH3COOH ϡ��ҺҪ�Ⱦ���С���ȴ�����ʹ�ã���е���Ϊ�˸�����Һ����Һ�е�_____�������ʵĻ�ѧʽ��
��2����Ʒ��ά���� C ����������Ϊ______���������
���𰸡�O2 92.4%
��������
(1)�������⣬����ά����C���н�ǿ�Ļ�ԭ�ԣ������ڿ������ױ�����������CH3COOH ϡ��Һ���ܽ���������Ϊ��֤ά����C�����ⶨ��ȷ�ԣ���������Ϊ�˳�ȥ��Һ����Һ��O2������ά����C��O2������
�ʴ�Ϊ��O2��
(2)�ζ����������ĵⵥ�ʵ����ʵ���=0.021L��0.05mol/L�����ݷ���ʽC6H8O6+ I2 = C6H6O6 +2HI��֪����Ʒ��ά����C�����ʵ���=n(I2)=0.021L��0.05mol/ L=0.00105mol��
����Ʒ��ά����C����������=![]() ��100%=92.4%��
��100%=92.4%��
����92.4%��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ˮ����ɱ�����������������ȷ����ˮ��ȫ�����������е�һƿ��84����Һ���İ�װ˵������������Ϣ����25%NaClO���������ƣ���1000mL���ܶ�1.19gcm-3��ϡ��100��������ȣ���ʹ�á�
��ش��������⣺
��1��������84����Һ�������ʵ���Ũ��Ϊ______molL-1��
��2����ͬѧȡ100mL������84����Һ����ϡ�ͺ�����������ϡ��100�������Һ��c��Na+��=______molL-1������ϡ�ͺ���Һ�ܶ�Ϊ1.0gcm-3����������Һ��ʱ������ڿ����������ձ�״����CO2�����Ϊ______L��
��3������־Ը�߸���������84����Һ���İ�װ˵��������NaClO���壨NaClO�����տ����е�H2O��CO2������480mL��25%NaClO������Һ������˵����ȷ����______��
a����ͼ��ʾ�������У��������Dz���Ҫ�ģ�����һ�ֲ�������
b������ƿ������ˮϴ����Ҫ��ɲ���������Һ������
c�����ù������ƷNaClO�����ƿ��ܻᵼ�½��ƫ��
d����ҪNaClO���������Ϊ143g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ��ˮֻ����ԭ������
A.2H2O+2Na=2NaOH+H2��
B.H2O + 3NO2 = 2HNO3 + NO
C.2H2O + 2F2 = O2 + 4HF
D.3H2O (��ˮ) + FeCl3![]() Fe (OH)3(����) + 3HCl
Fe (OH)3(����) + 3HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ƾ��壨![]() ���������մ�����������ˮ���������Ҵ��������Ի������Һ���ȶ����㷺Ӧ�����ճ����������С��ش��������⣺
���������մ�����������ˮ���������Ҵ��������Ի������Һ���ȶ����㷺Ӧ�����ճ����������С��ش��������⣺
I.��������ƵĽṹ������
��1��![]() �Ľṹʽ��ͼ��ʾ������
�Ľṹʽ��ͼ��ʾ������![]() �Ļ��ϼ�Ϊ____��
�Ļ��ϼ�Ϊ____��

��2��![]() ���������������ʲ��ȶ���ȡ����
���������������ʲ��ȶ���ȡ����![]() ��Һ���Թ��У���������6
��Һ���Թ��У���������6![]() �����ᣬ������Ӧ�����ӷ���ʽΪ____��
�����ᣬ������Ӧ�����ӷ���ʽΪ____��
II.��������ƾ�����Ʊ�
��3����Բ����ƿ�м���12g![]() ��60mLˮ��4g��ƣ�����1Сʱ���ȼ�ѹ���ˣ������Ҵ�ϴ�Ӿ��塢�����õ�
��60mLˮ��4g��ƣ�����1Сʱ���ȼ�ѹ���ˣ������Ҵ�ϴ�Ӿ��塢�����õ�![]() ���塣
���塣
��д���Ʊ�![]() �Ļ�ѧ����ʽ��____��
�Ļ�ѧ����ʽ��____��
�����Ҵ�ϴ�Ӿ����ԭ����________��
III.![]() ���庬���IJⶨ
���庬���IJⶨ
��4��ȷ��ȡ1.5g��Ʒ������20mL��в���ȴ���ˮʹ����ȫ�ܽ⣬�Ե�����ָʾ������0.1000 ![]() ��ı���Һ�ζ�����֪��
��ı���Һ�ζ�����֪��![]() ����ɫ��+
����ɫ��+![]() ���������ˮ����Ӧ��
���������ˮ����Ӧ��
�ٵ�ı���ҺӦʢ����____�����ʽ����ʽ�����ζ����С�
���жϵζ��յ������Ϊ____��
�۵�һ�εζ���ʼ�ͽ���ʱ���ζ����е�Һ����ͼ��ʾ�����һ�����ĵ�ı���Һ�����Ϊ____mL��

���ظ������������Σ���¼�������±������Ʒ�еĺ���Ϊ____%���������1λС������
�ζ����� | �ζ�ǰ����/mL | �ζ������/mL |
�ڶ��� | 1.56 | 30.30 |
������ | 0.22 | 26.31 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͨ������£�pH<7 ����Һ�����ԣ�pH=7 ����Һ�����ԣ�pH>7 ����Һ�Լ��ԡ��� FeCl3 ��ҺΪʵ�����̽�������������֮�䷴Ӧ�ĸ��Ӷ����ԡ�ʵ�����£�
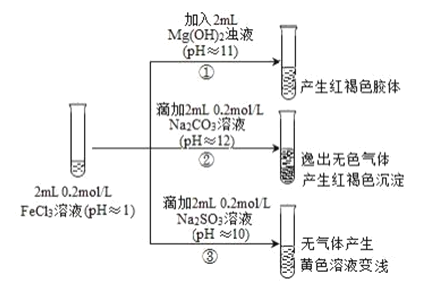
��֪���� Fe2+����Һ�м��� K3Fe(CN)6 ��Һ������ɫ������K3Fe(CN)6 = 3K++Fe(CN)6 3-
��1�����з�Ӧ�����ӷ���ʽ��______��
��2��д�����з�����Ӧ�Ļ�ѧ����ʽ______��
��3���������е�ʵ������ͬѧ�������²⣬��������ʵ�飺
���飺ȡ���з�Ӧ����Һ����������ϡ�����ữ���ٵμ� BaCl2 ��Һ��������ɫ�������ó����ۣ�FeCl3 �� Na2SO3 ������������ԭ��Ӧ������SO32-������������______�������ӵĻ�ѧʽ����
���飺��Ϊ�����ʵ�鲻�Ͻ���������Ʋ�����ʵ�飬֤ʵ�˼���Ľ�������ȷ�ġ���ʵ�鷽����ȡ���з�Ӧ�����Һ������ K3Fe(CN)6 ��Һ������______������ɫ�����Ļ�ѧʽ�������в�����Ԫ�أ���˵�������� Fe2+����д�� FeCl3 �� Na2SO3 ��Һ��Ӧ�����ӷ���ʽ��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ϡ��Ԫ����Ԫ�����ڱ��е�IIIB���֡��ƺ���ϵԪ�ص��ܳơ���������������ϡ���������NdFeB������������ۺϴ����ܣ����㷺Ӧ���ڼ������ͨ����Ϣ�ȸ��¼�����ҵ���ش��������⣺
��1����̬Feԭ�ӵļ۵����Ų�ʽΪ____�������ܡ���Ԫ�����ʷdz����ƣ�ԭ�Ӱ뾶�ӽ������μ�С��NiO��FeO�ľ���ṹ�������Ȼ�����ͬ������NiO____���>����<����=����FeO��
��2�������黯���![]() ����һ�����ͻ�ѧ������ϣ���û�������ӻ�Ϊ�ȵ�������л���Ϊ___���ѧʽ���������������N��Bԭ�ӵ��ӻ���ʽ�ֱ�Ϊ___��___��
����һ�����ͻ�ѧ������ϣ���û�������ӻ�Ϊ�ȵ�������л���Ϊ___���ѧʽ���������������N��Bԭ�ӵ��ӻ���ʽ�ֱ�Ϊ___��___��
��3��![]() �׳�Ħ���Σ������
�׳�Ħ���Σ������![]() ���ԣ�Ħ���β���ʧˮ�����ױ������������ڻ�ѧ����ʵ���г���������Fe��II���ı���Һ���Է�����������茶��������������ȶ����ڵ�ԭ��______
���ԣ�Ħ���β���ʧˮ�����ױ������������ڻ�ѧ����ʵ���г���������Fe��II���ı���Һ���Է�����������茶��������������ȶ����ڵ�ԭ��______
��4����������õ�ϡ������֮һ������Ϊ������ϵ����ԭ�����������ܶѻ���ʽ���ӡ�����������![]() ��ÿ����������___����ԭ�ӣ��谢���ӵ�����Ϊ
��ÿ����������___����ԭ�ӣ��谢���ӵ�����Ϊ![]() ��������ϵ��ܶ�Ϊ___
��������ϵ��ܶ�Ϊ___![]() ��Nd�����ԭ������ΪM���г��������ʽ��
��Nd�����ԭ������ΪM���г��������ʽ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NA���������ӵ�������ֵ�������й�������ȷ����
A. ��״���£�5.6L һ��������5.6L ������Ϻ�ķ�������Ϊ0.5NA
B. �������Ũ�Ⱦ�Ϊ1mol/L����������ᣬ���������������֮��Ϊ3:1
C. һ���¶��£�1L 0.50 mol/L NH4Cl��Һ��2L 0.25 mol/L NH4Cl��Һ��NH4+�����ʵ�����ͬ
D. ��״���£��������N2��CO������ԭ������Ϊ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ۺ�����CO2��CO�Թ�����̼�������Ҫ���塣
��1��H2 ��CO�ϳɼ״���ӦΪ��CO��g��+2H2��g��![]() CH3OH��g�� ��H��0���ں��£����Ϊ2L���ܱ������зֱ����1.2mol CO��1mol H2��10min��ﵽƽ�⣬��ú���0.4mol CH3OH��g������ﵽƽ��ʱCO��Ũ��Ϊ_______��10min����H2��ʾ�Ļ�ѧ��Ӧ����Ϊ_______����Ҫ�ӿ�CH3OH���������ʲ����CO��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��_______����һ�ֺ����Ĵ�ʩ����
CH3OH��g�� ��H��0���ں��£����Ϊ2L���ܱ������зֱ����1.2mol CO��1mol H2��10min��ﵽƽ�⣬��ú���0.4mol CH3OH��g������ﵽƽ��ʱCO��Ũ��Ϊ_______��10min����H2��ʾ�Ļ�ѧ��Ӧ����Ϊ_______����Ҫ�ӿ�CH3OH���������ʲ����CO��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��_______����һ�ֺ����Ĵ�ʩ����
��2��������̼�ϳɼ״���̼���ŵ��·���CO2ת��Ϊ�״����Ȼ�ѧ����ʽΪ��CO2(g) +3H2(g) ![]() CH3OH(g) +H2O(g) ��H
CH3OH(g) +H2O(g) ��H
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪK=________��
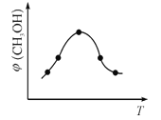
���ں����ܱ�������ʹCO2��H2�����ʵ���֮��Ϊ1��3��,����������Ӧ����Ӧ�����в�ü״������������(CH3OH)�뷴Ӧ�¶�T�Ĺ�ϵ����ͼ��ʾ���� ��H _________0����������������С������
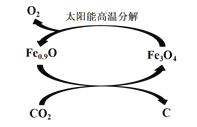
��3�� ����ͼ��ʾ������ȱ��������[��Fe0.9O]��ʵ��CO2���ۺ����á���˵����ת����2���ŵ�_____________������1 molȱ��������[Fe0.9O]������CO2��ȫ��Ӧ������________mol C(̼)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���ܱ��������м���һ�����ɻ����ĸ���(��ȿɺ���)�������ֳ������֣���������1 mol N2���Ҳ����CO��CO2�Ļ�����干8 gʱ�����崦����ͼλ��(���������¶���ͬ)������˵����ȷ����
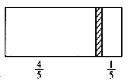
A. �Ҳ�CO��CO2������֮��Ϊ1��3
B. �Ҳ������ܶ�����ͬ�����������ܶȵ�18��
C. �Ҳ�CO������Ϊ1.75 g
D. �����崦�ھ����Ҷ�1/6���������������䣬��ǰ������ѹǿ֮��Ϊ25��24
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com